Continuing Education Activity
Dural arteriovenous fistulas, sometimes referred to as dural arteriovenous malformations, are vascular abnormalities in which arteries arising from branches of the carotid or vertebral arteries drain directly into the dural leaflets of the venous sinuses. They are more commonly supratentorial than infratentorial, and the transverse-sigmoid junction is the most common location with a slight left-sided predominance. This activity outlines how to properly evaluate dural arteriovenous fistulas, and highlights the role of the interprofessional team in caring for patients with this condition.
Objectives:
- Outline the utility of the Borden and Cognard classifications for dural arteriovenous fistulas.
- Describe the presentation of dural arteriovenous fistulas.
- Review the treatment and dural arteriovenous fistulas.
- Explain how the facilitation of interprofessional team education and discussion can optimize the effective detection of dural arteriovenous fistulas and inform the need for subsequent evaluations.
Introduction
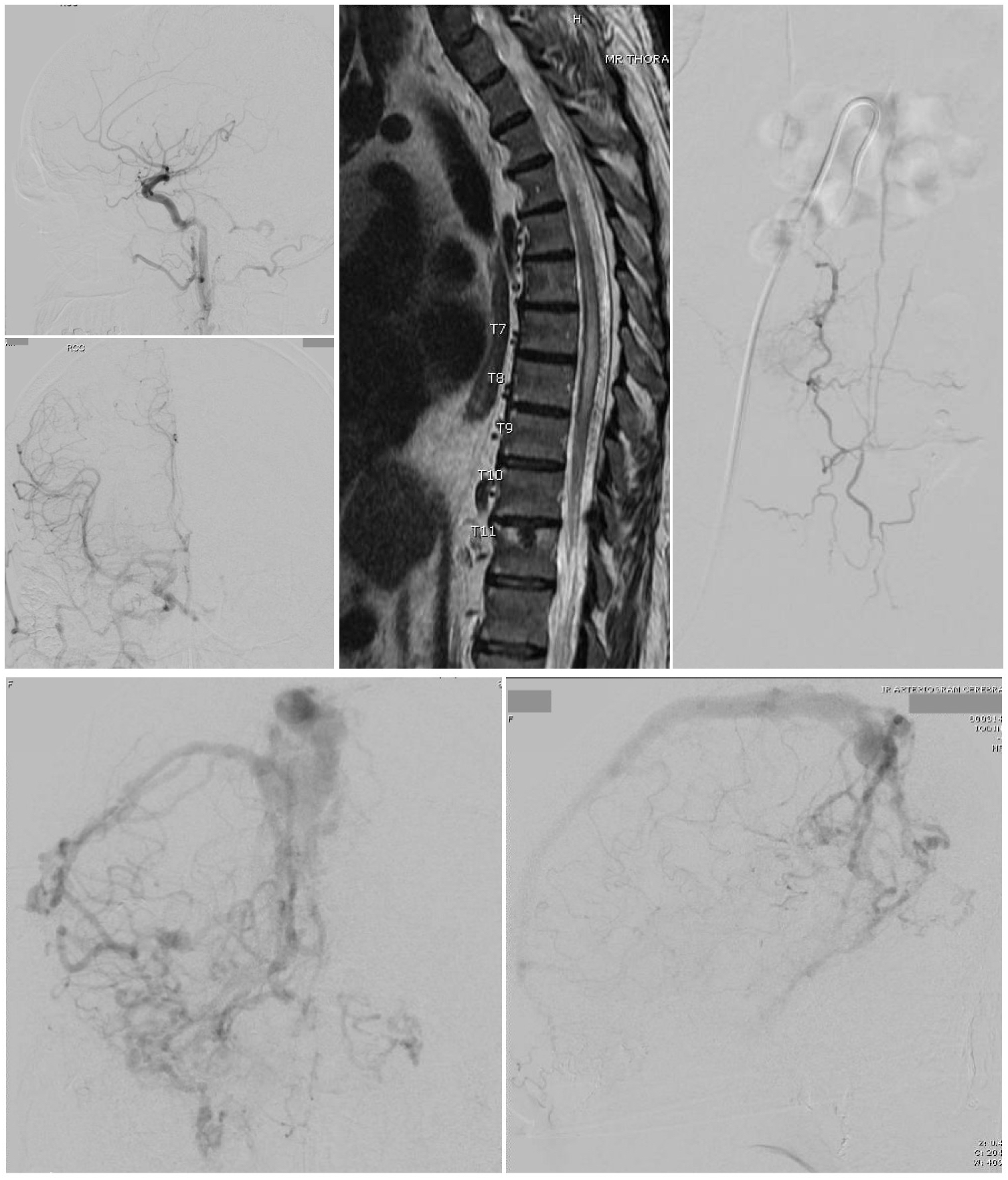
Dural arteriovenous fistulas (dAVF) are vascular abnormalities in which arteries arising from branches of the carotid or vertebral arteries drain directly into the dural leaflets of the venous sinuses. They are sometimes referred to as dural arteriovenous malformations. Their location is more commonly supratentorial than infratentorial. The transverse-sigmoid junction is the most common location, with a slight left-sided predominance. They can also be found at tentorial, petrosal, ethmoidal, Sylvian, cavernous sinus, spinal dura, and superior sagittal sinus locations. When located in the cavernous sinus, they are referred to as carotid-cavernous fistulas.[1][2]
The clinical behavior of dAVFs, including the risk of intracranial hypertension and hemorrhage, predominantly depends on the venous drainage patterns. Cortical venous drainage predisposes to a more aggressive clinical course. Tentorial location is associated with most aggressive behavior, followed by Sylvian/middle fossa and ethmoidal/anterior fossa locations.[3] The Borden and Cognard classifications are the most well-known classification systems used for predicting the aggressiveness of dAVFs.[2][4]
Etiology
Most dural arteriovenous fistulas have no clear origin; however, there is evidence that many are caused after a dural sinus thrombosis, trauma, infection, or prior craniotomy.[5] Those dAVFs involving the larger brain veins usually arise from progressive narrowing or blockage of one of the brain's venous sinuses, which route circulated blood from the brain back to the heart.
Epidemiology
Incidence is approximately 0.15 - 0.29 per 100,000 persons per year.[6][7] Dural arteriovenous fistulas can present at any age but are most commonly diagnosed between the ages of 40 and 60.[2][8] However, dAVFs can occur in younger age groups as well, including in children. The dAVFs account for 10-15% of all cerebral vascular malformations.[8]
Pathophysiology
The main abnormality in dAVFs is a connection between the dural arteries and veins within the venous sinus wall via small vessels that are approximately 30 micrometers on average.[9] In those patients with a provoking event, neovascularization is induced by a previously thrombosed dural venous sinus, typically the transverse sinus.
Patients with idiopathic fistula usually have prior asymptomatic thrombosis of a dural venous sinus, secondary to inherited prothrombotic conditions (antithrombin, protein C deficiency, and protein S deficiency) or systemic illness or diseases producing prothrombotic conditions.[8]
History and Physical
History and physical exam should include a thorough neurological examination as well as an evaluation of the overall medical status for surgical risk stratification.
Some people with a dAVF may not have any symptoms and they are discovered during brain neuroimaging studies in the workup for other conditions. Some dAVFs can remain asymptomatic for a long period of time or even involute spontaneously[10]
Those with symptoms can be characterized either as aggressive or benign and can include any or a combination of the following symptoms:[2][8][10]
- Headache
- Nausea/vomiting
- Seizures
- Cranial neuropathies
- Pulsatile tinnitus (bruits)
- Intracranial hypertension
- Papilledema
- Glaucoma
- Hydrocephalus
- Intracerebral hemorrhage
- Speech or language issues
- Coordination issues
- Altered sensations
- Weakness
- Face pain
- Dementia
- Parkinsonism
- Apathy
- Vision problems
- Proptosis
Evaluation
Imaging studies in which dAVFs may be identified are computed tomography angiography (CTA) or magnetic resonance angiography (MRA), demonstrating dilated tortuous vessels corresponding to abnormal arteries and veins, sinus enlargement, or sinus occlusion. In addition, MRA may reveal associated early prominent sinus filling, dilated pial vessels, and associated edema from venous hypertension seen as T2-weighted hyperintensity. This venous hypertension leptomeningeal edema may occur in up to half of the patients even if they do not have a cortical retrograde filling. Hydrocephalus can also be identified if there is hypertension in the venous sinuses.[10]
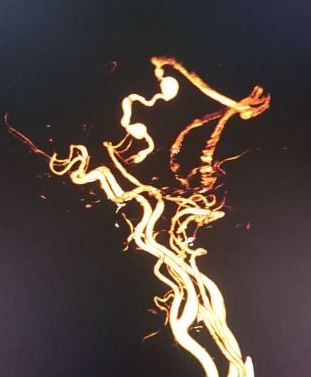
A six-vessel cerebral digital subtraction angiogram (DSA) is required to establish the diagnosis and plan treatment. It defines the fistula, the external carotid circulation involvement, sinus patency or blockage, and affected dilated veins.
The Borden classification system describes lesions as the following types based on the direction of flow and presence of cortical venous drainage:[4]
- Type I: anterograde flow into a dural venous sinus or meningeal vein. Usually have a benign natural history.
- Type II: anterograde flow into a dural venous sinus. However, they also have retrograde cortical venous reflux. These are considered high-grade lesions with aggressive behavior in 39%.
- Type III: direct retrograde flow from the fistula into cortical veins, thereby causing venous hypertension. They have aggressive behavior in 79%.
The Cognard classification, dAVFs are divided into the following seven categories based on the location, the direction of flow, presence of cortical venous drainage, and presence of venous ectasia:[11]
- Type I: confined to sinus, antegrade flow, and no cortical drainage. They have a benign clinical course.
- Type IIa: confined to the sinus, retrograde flow into the sinus, no cortical drainage. These have a 20% risk of intracranial hypertension.
- Type IIb: drainage into a venous sinus, anterograde flow, reflux into cortical veins. These have a 10% risk of hemorrhage induced by venous reflux.
- Type IIa+b: drainage into a venous sinus, retrograde flow, reflux into cortical veins. These have a 66% risk of hemorrhage with or without intracranial hypertension.
- Type III: direct drainage into a cortical vein without venous ectasia. These have a 40% risk of hemorrhage.
- Type IV: direct drainage into a cortical vein with venous ectasia. These have a 65% risk of hemorrhage.
- Type V: direct drainage into spinal perimedullary veins. They present with progressive myelopathy in 50% of cases.
Treatment / Management
The decision of whether to treat dAVFs is based on the patient's symptoms, medical comorbidities, and risk of intracranial hypertension or hemorrhage. Lesions that are asymptomatic and low-grade tend to have a benign natural history and are generally managed conservatively with serial monitoring.[10] Those that are high-grade with cortical venous drainage or symptomatic are candidates for an intervention. Open surgery, endovascular embolization, and stereotactic radiosurgery are the main options for intervention. The goal of treatment is to achieve a complete disconnection of the fistula from its venous drainage. Incomplete disconnection may allow for the recruitment of other arteries to the fistula and may not improve the risk of hemorrhage or symptoms.[12]
Surgical treatment involves the surgical disconnection of the fistula from the cortical venous system. Occasionally, skeletonization of the dural sinus with disconnection of the dural arterial supply, packing of the sinus, or resection of the involved dura is performed. Surgery is usually indicated in cases in which endovascular approaches have failed or can not be performed. In addition, those involving the floor of the anterior cranial fossa are usually treated surgically.
Endovascular surgery using embolic material or coil occlusion can involve transvenous, trans-arterial, direct access, or a combination of techniques to block the abnormal connection in the blood vessels.[12] The treatment is aimed at the complete elimination of the arteriovenous shunt. This can be performed transarterial or transvenous.
Stereotactic radiosurgery achieves excellent rates of obliteration for low-grade lesions but is less effective for higher-grade lesions. Radiosurgery is most often utilized for low-grade (Borden type I) dAVFs with persistent symptoms such as pulsatile tinnitus. It can also be used for dAVFs with unfavorable anatomy for other interventions, patients with significant medical comorbidities, or as salvage therapy for lesions incompletely treated with surgery or endovascular embolization.[12][13] The area is irradiated with 20–30 Gy, which causes vessel thrombosis and fistula closure. The end result takes several months to various years with a risk of hemorrhage until final obliteration.
Differential Diagnosis
- Cerebral arteriovenous malformations
- Cavernous malformations
- Highly vascular tumors
Prognosis
The outcome depends on the degree of neurological deficit and symptoms. In most patients, the pulsatile tinnitus can be cured by closing the fistula. Both the seizures and visual problems can improve significantly after therapy.[8] Stroke cannot be cured, but any new neurological deficit can be prevented. Areas of edema and hypertension will improve and resolve after the fistula is obliterated and there is no more retrograde cortical drainage. Both surgery and the endovascular approach do have their own complications, which can add to the morbidity. Radiosurgery can also cause complications in which radiation necrosis is the most devastating.[13]
Higher grades (Borden types II and III, Cognard types IIb-V) have an annual mortality rate of approximately 10% and an annual risk of intracranial hemorrhage of approximately 8%. Non-hemorrhagic neurological deficits have an annual risk of approximately 7%.
Complications
- Intracerebral hemorrhage
- Subdural hematomas
- Subarachnoid hemorrhage
- Increased intracranial pressure
- Seizures
Consultations
- Neurosurgery
- Neuroradiologist
- Endovascular neurosurgeon or neuroradiologist
- Physiatrist
- Intensivist
- Neurologist
Deterrence and Patient Education
The presence of a dural arteriovenous fistula can cause significant physical and mental health effects in the patient. These lesions can severely affect the quality of life and the lifespan of an affected patient. Many patients present with intracerebral hemorrhages, affecting their mobility and verbal communication depending on the location and size. Patients need to be well-oriented about the DSA results and the natural history of their particular fistula in terms of hemorrhage risk, so they can make informed decisions. Patients should be oriented that ischemic injuries are permanent, but treatment will prevent future damage. Physical therapy, occupational therapy, and speech therapy are needed for those with permanent deficits. In those patients where the dAVF is not treated initially, close follow-up is necessary to assess the development of new symptoms or progression of existing ones.
Enhancing Healthcare Team Outcomes
Dural arteriovenous fistulas are not common, and hence there are no universal guidelines for clinicians to follow. The clinical signs of the condition are heterogeneous, and a high index of suspicion is necessary for the diagnosis. An integrated approach with a team of healthcare professionals is recommended to make an early diagnosis and appropriate treatment. The neurologist, neurosurgeon, interventional radiologist, and critical care physician can coordinate the treatment care plan and monitoring.
Post-treatment, the nurse is vital for monitoring the neurological exam and helping with ambulation and feeding. The nurse also plays a role in educating the patient and family about the disease and the potential adverse effects of treatment. In addition, the pharmacist ensures that the patient remains compliant with medications and controls his or her blood pressure.