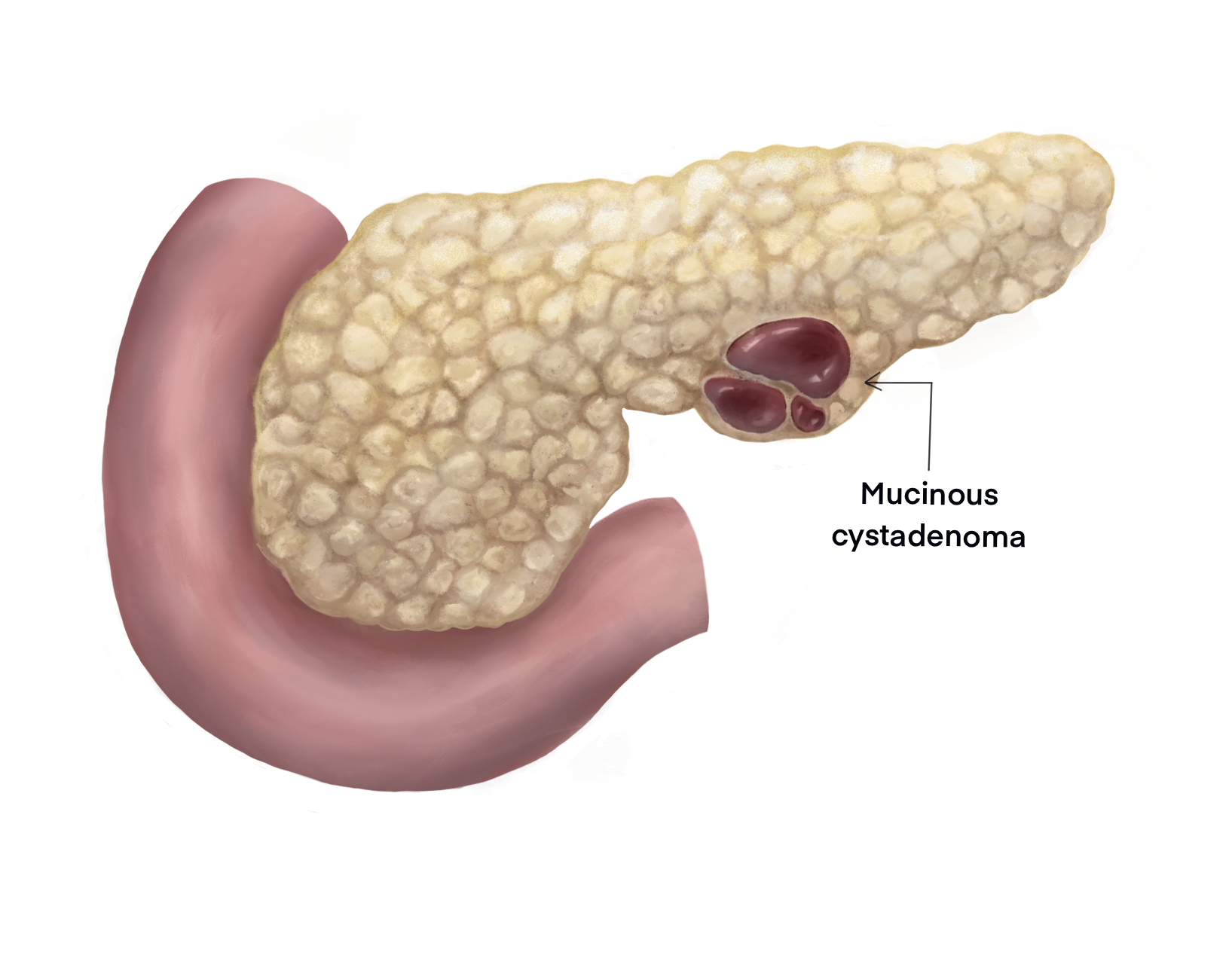
[1]
Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. American journal of clinical pathology. 1978 Jun:69(6):573-80
[PubMed PMID: 665578]
Level 3 (low-level) evidence
[2]
Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S, International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2006:6(1-2):17-32
[PubMed PMID: 16327281]
Level 3 (low-level) evidence
[3]
Wenig BM, Albores-Saavedra J, Buetow PC, Heffess CS. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. The American journal of surgical pathology. 1997 Jan:21(1):70-80
[PubMed PMID: 8990143]
Level 3 (low-level) evidence
[4]
Zamboni G,Scarpa A,Bogina G,Iacono C,Bassi C,Talamini G,Sessa F,Capella C,Solcia E,Rickaert F,Mariuzzi GM,Klöppel G, Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. The American journal of surgical pathology. 1999 Apr;
[PubMed PMID: 10199470]
[5]
Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Annals of surgery. 1999 Aug:230(2):152-61
[PubMed PMID: 10450728]
Level 2 (mid-level) evidence
[6]
Garcea G, Ong SL, Rajesh A, Neal CP, Pollard CA, Berry DP, Dennison AR. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2008:8(3):236-51. doi: 10.1159/000134279. Epub 2008 May 23
[PubMed PMID: 18497542]
[7]
Fernández-del Castillo C, Warshaw AL. Cystic tumors of the pancreas. The Surgical clinics of North America. 1995 Oct:75(5):1001-16
[PubMed PMID: 7660245]
[8]
Reddy RP,Smyrk TC,Zapiach M,Levy MJ,Pearson RK,Clain JE,Farnell MB,Sarr MG,Chari ST, Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004 Nov;
[PubMed PMID: 15551256]
[9]
Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Annals of surgery. 2000 Feb:231(2):205-12
[PubMed PMID: 10674612]
[10]
Testini M, Gurrado A, Lissidini G, Venezia P, Greco L, Piccinni G. Management of mucinous cystic neoplasms of the pancreas. World journal of gastroenterology. 2010 Dec 7:16(45):5682-92
[PubMed PMID: 21128317]
[11]
Gress F, Gottlieb K, Cummings O, Sherman S, Lehman G. Endoscopic ultrasound characteristics of mucinous cystic neoplasms of the pancreas. The American journal of gastroenterology. 2000 Apr:95(4):961-5
[PubMed PMID: 10763945]
[12]
Waters JA,Schmidt CM,Pinchot JW,White PB,Cummings OW,Pitt HA,Sandrasegaran K,Akisik F,Howard TJ,Nakeeb A,Zyromski NJ,Lillemoe KD, CT vs MRCP: optimal classification of IPMN type and extent. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008 Jan;
[PubMed PMID: 17917784]
[13]
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004 May:126(5):1330-6
[PubMed PMID: 15131794]
[14]
European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018 May:67(5):789-804. doi: 10.1136/gutjnl-2018-316027. Epub 2018 Mar 24
[PubMed PMID: 29574408]
Level 1 (high-level) evidence
[15]
Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. The American journal of gastroenterology. 2018 Apr:113(4):464-479. doi: 10.1038/ajg.2018.14. Epub 2018 Feb 27
[PubMed PMID: 29485131]
[16]
Lee SY,Allen PJ,Sadot E,D'Angelica MI,DeMatteo RP,Fong Y,Jarnagin WR,Kingham TP, Distal pancreatectomy: a single institution's experience in open, laparoscopic, and robotic approaches. Journal of the American College of Surgeons. 2015 Jan;
[PubMed PMID: 25456783]
[17]
Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, Hruban RH. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. The American journal of surgical pathology. 1999 Nov:23(11):1320-7
[PubMed PMID: 10555000]
[18]
Hackert T, Werner J, Büchler MW. Postoperative pancreatic fistula. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2011 Aug:9(4):211-7. doi: 10.1016/j.surge.2010.10.011. Epub 2010 Dec 13
[PubMed PMID: 21672661]