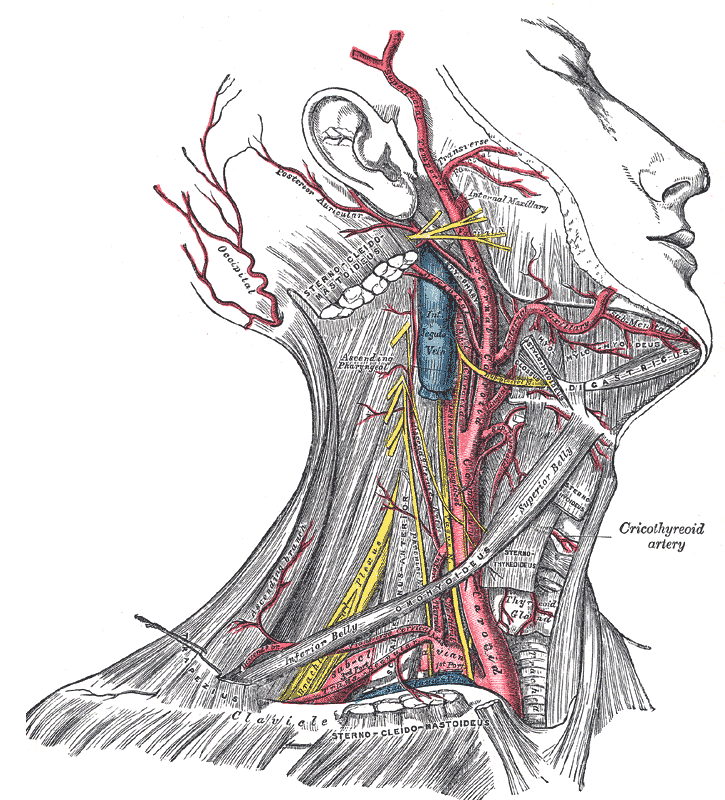
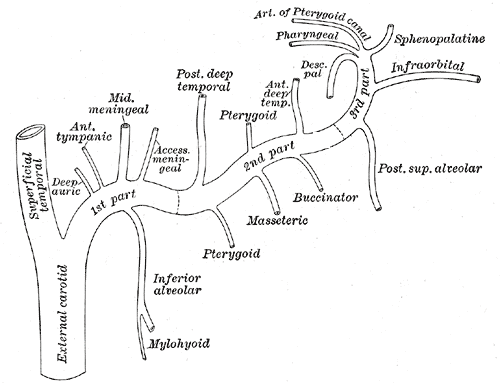
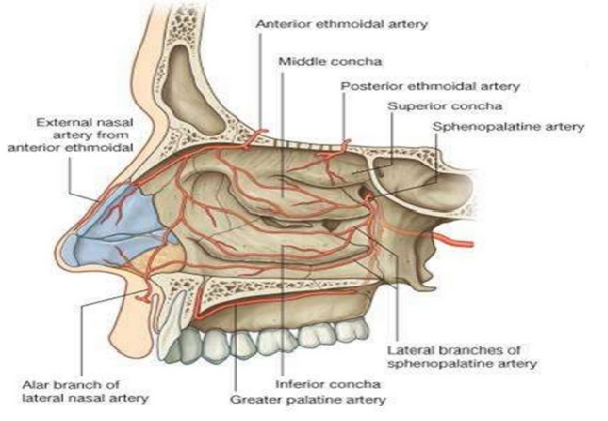
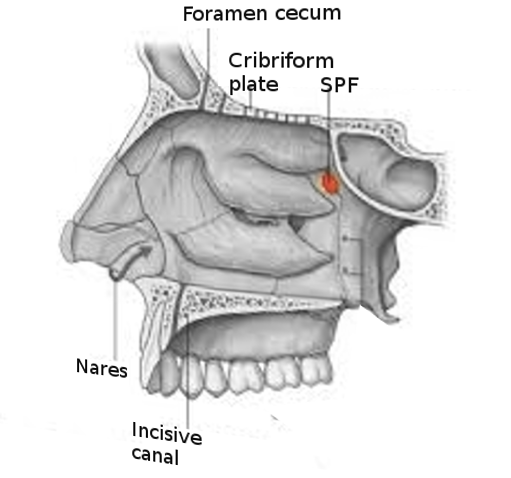
[1]
Small M, Murray JA, Maran AG. A study of patients with epistaxis requiring admission to hospital. Health bulletin. 1982 Jan:40(1):20-9
[PubMed PMID: 7061227]
[2]
Sireci F, Speciale R, Sorrentino R, Turri-Zanoni M, Nicolotti M, Canevari FR. Nasal packing in sphenopalatine artery bleeding: therapeutic or harmful? European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2017 Mar:274(3):1501-1505. doi: 10.1007/s00405-016-4381-y. Epub 2016 Nov 11
[PubMed PMID: 27837422]
[3]
Gras-Cabrerizo JR, Ademá-Alcover JM, Gras-Albert JR, Kolanczak K, Montserrat-Gili JR, Mirapeix-Lucas R, Del Campo FS, Massegur-Solench H. Anatomical and surgical study of the sphenopalatine artery branches. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2014 Jul:271(7):1947-51. doi: 10.1007/s00405-013-2825-1. Epub 2013 Nov 20
[PubMed PMID: 24253386]
[4]
Gras-Cabrerizo JR, García-Garrigós E, Montserrat-Gili JR, Gras-Albert JR, Mirapeix-Lucas R, Massegur-Solench H, Quer-Agusti M. Anatomical Correlation Between Nasal Vascularisation and the Design of the Endonasal Pedicle Flaps. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2018 Mar:70(1):167-173. doi: 10.1007/s12070-017-1197-z. Epub 2017 Sep 6
[PubMed PMID: 29456964]
[5]
Tanoue S, Kiyosue H, Mori H, Hori Y, Okahara M, Sagara Y. Maxillary artery: functional and imaging anatomy for safe and effective transcatheter treatment. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Nov-Dec:33(7):e209-24. doi: 10.1148/rg.337125173. Epub
[PubMed PMID: 24224604]
[6]
El-Shaarawy EAA, Hassan SS. The sphenopalatine foramen in man: anatomical, radiological and endoscopic study. Folia morphologica. 2018:77(2):345-355. doi: 10.5603/FM.a2017.0104. Epub 2017 Nov 13
[PubMed PMID: 29131280]
[7]
Eordogh M, Grimm A, Gawish I, Patonay L, Reisch R, Briner HR, Baksa G. Anatomy of the sphenopalatine artery and its implications for transnasal neurosurgery. Rhinology. 2018 Mar 1:56(1):82-88. doi: 10.4193/Rhin17.181. Epub
[PubMed PMID: 29166425]
[8]
Krulewitz NA, Fix ML. Epistaxis. Emergency medicine clinics of North America. 2019 Feb:37(1):29-39. doi: 10.1016/j.emc.2018.09.005. Epub
[PubMed PMID: 30454778]
[9]
Gibelli D, Cellina M, Gibelli S, Cappella A, Panzeri MM, Oliva AG, Termine G, Dolci C, Sforza C. Anatomy of the pterygopalatine fossa: an innovative metrical assessment based on 3D segmentation on head CT-scan. Surgical and radiologic anatomy : SRA. 2019 May:41(5):523-528. doi: 10.1007/s00276-018-2153-7. Epub 2018 Dec 12
[PubMed PMID: 30542926]
[11]
Plzák J, Kratochvil V, Kešner A, Šurda P, Vlasák A, Zvěřina E. Endoscopic endonasal approach for mass resection of the pterygopalatine fossa. Clinics (Sao Paulo, Brazil). 2017 Oct:72(9):554-561. doi: 10.6061/clinics/2017(09)06. Epub
[PubMed PMID: 29069259]
[12]
Konno A, Togawa K. Role of the vidian nerve in nasal allergy. The Annals of otology, rhinology, and laryngology. 1979 Mar-Apr:88(2 Pt 1):258-66
[PubMed PMID: 443720]
[14]
Simmen DB, Raghavan U, Briner HR, Manestar M, Groscurth P, Jones NS. The anatomy of the sphenopalatine artery for the endoscopic sinus surgeon. American journal of rhinology. 2006 Sep-Oct:20(5):502-5
[PubMed PMID: 17063746]
[15]
Lee HY, Kim HU, Kim SS, Son EJ, Kim JW, Cho NH, Kim KS, Lee JG, Chung IH, Yoon JH. Surgical anatomy of the sphenopalatine artery in lateral nasal wall. The Laryngoscope. 2002 Oct:112(10):1813-8
[PubMed PMID: 12368621]
[16]
Barger J, Siow M, Kader M, Phillips K, Fatterpekar G, Kleinberg D, Zagzag D, Sen C, Golfinos JG, Lebowitz R, Placantonakis DG. The posterior nasoseptal flap: A novel technique for closure after endoscopic transsphenoidal resection of pituitary adenomas. Surgical neurology international. 2018:9():32. doi: 10.4103/sni.sni_192_17. Epub 2018 Feb 14
[PubMed PMID: 29527390]
[17]
Reuter G, Bouchain O, Demanez L, Scholtes F, Martin D. Skull base reconstruction with pedicled nasoseptal flap: Technique, indications, and limitations. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2019 Jan:47(1):29-32. doi: 10.1016/j.jcms.2018.11.012. Epub 2018 Nov 16
[PubMed PMID: 30527383]
[18]
Iimura J, Hatano A, Ando Y, Arai C, Arai S, Shigeta Y, Kojima H, Otori N, Wada K. Study of hemostasis procedures for posterior epistaxis. Auris, nasus, larynx. 2016 Jun:43(3):298-303. doi: 10.1016/j.anl.2015.09.015. Epub 2015 Oct 31
[PubMed PMID: 26527519]
[19]
Bakshi SS, Bhattacharjee S. Juvenile Nasopharyngeal Angiofibroma. Journal of pediatric hematology/oncology. 2016 Aug:38(6):491-2. doi: 10.1097/MPH.0000000000000568. Epub
[PubMed PMID: 27164528]
[20]
Makhasana JA, Kulkarni MA, Vaze S, Shroff AS. Juvenile nasopharyngeal angiofibroma. Journal of oral and maxillofacial pathology : JOMFP. 2016 May-Aug:20(2):330. doi: 10.4103/0973-029X.185908. Epub
[PubMed PMID: 27601836]