Continuing Education Activity
Papillary fibroelastomas are one of the most common types of primary cardiac tumors. They are increasingly recognized as the spatial resolution of imaging modalities improves. Also, they are a potential cause of transient ischemic attacks, strokes, myocardial infarction, and sudden death. This activity reviews the evaluation and treatment of papillary fibroelastomas and highlights the role of the cardiac team in managing the care of patients with this condition.
Objectives:
Identify the pathology of papillary fibroelastomas.
Review the evaluation of papillary fibroelastomas.
Outline the treatment and management options available for papillary fibroelastomas.
Summarize interprofessional team strategies for improving care coordination and communication to advance papillary fibroelastoma treatment and improve outcomes.
Introduction
Primary cardiac tumors are rare entities, and the majority of these are benign. In adults, papillary fibroelastomas (PFEs) and cardiac myxomas are the most common types of primary cardiac tumors. Papillary fibroelastoma is a benign neoplasm. A single large series of 511 patients at Mayo Clinic by Tamin et al. reported PFEs as the most common cardiac tumor [1]. Increasing utilization of advanced imaging techniques such as transesophageal echocardiography (TEE) has led to more frequent identification of intracardiac tumors.[1] The first reported case of PFE was in 1975 as an embolic complication of PFE, leading to myocardial infarction.[2] Since then, papillary fibroelastomas have been identified as a possible etiology of vascular embolism, stroke, and cardiac arrest. Much controversy still exists in the surgical versus medical management of these “benign” tumors, as they are known to lead to downstream embolic complications. This activity will focus on providing the latest literature update on PFEs.
Etiology
The precise etiology of papillary fibroelastomas remains largely unknown as they are relatively rare tumors. They originate mostly from the valvular endocardium, as the belief is it arises in areas of valvular endothelial damage.
Epidemiology
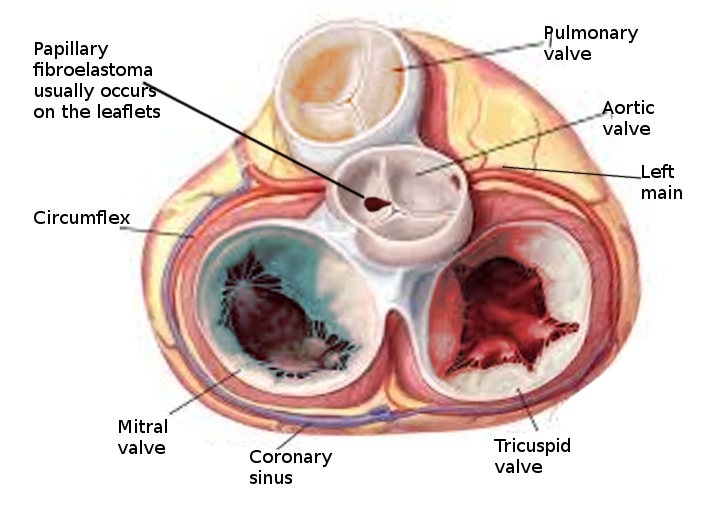
The incidence of primary cardiac tumors is extremely rare, with one study identifying an incidence of less than 0.1 %[3]. Two large studies showed that about 55% of affected patients were male, and the mean age at detection was 60 years. Because many PFEs are only identified post-mortem, the true incidence is unknown. The most common location of PFE is the aortic valve (35% to 63%), but other valves are commonly involved, including the mitral valve (9% to 55%), tricuspid valve (6%-15%), and pulmonic valve (0.5% to 8%).[4][1][5]
Pathophysiology
Research has yet to identify a precise pathophysiologic mechanism. There are, however, several theories postulated on the pathophysiology of PFEs. One oft-mentioned hypothesis is that these lesions are an acquired entity and that they begin as microthrombi. These microthrombi join at sites of minor endothelial damage on valvular surfaces. Over time, these microthrombi evolve into excrescences and, eventually, PFEs.[6] These PFEs continue to grow and can potentially embolize, causing resultant complications, including stroke, myocardial infarction, ventricular fibrillation, and sudden death. The most commonly affected arterial beds from embolization are cerebral arteries leading to a spectrum from transient ischemic attacks to stroke.[7]
Histopathology
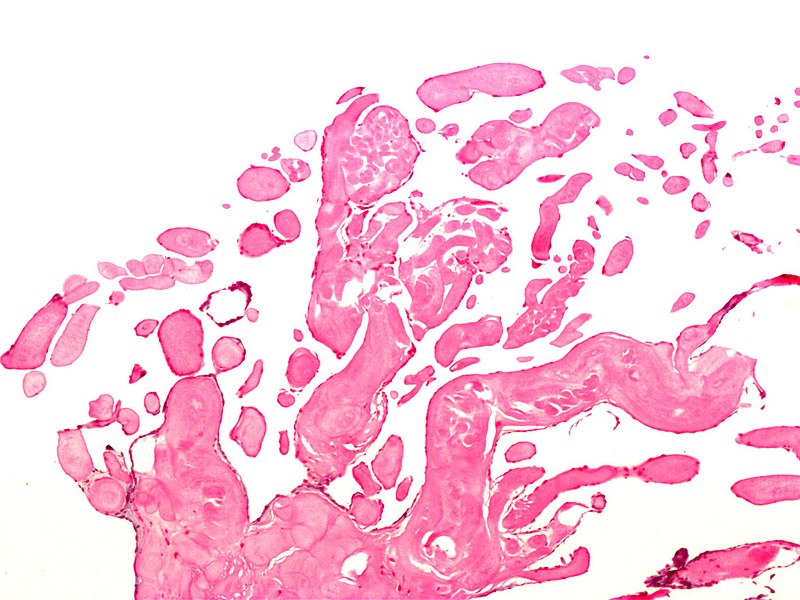
The gross appearance of papillary fibroelastoma is classically described as a "sea anemone" with a central stalk and frond-like arms projecting outward. In vivo, these tumors can be as small as 2 mm with a mean of 9 mm and range up to several cm in maximal dimension. Histologically, the composition of PFEs is primarily collagen, elastin, and reticulin, with very little vasculature.[5] The outer layer is comprised of the endothelium, an intermediate later with mucopolysaccharide-rich connective tissue, and a central core of fibrin and mucopolysaccharide.
History and Physical
Papillary fibroelastoma is most commonly asymptomatic and discovered incidentally. In some patients, PFE first manifests with features of embolization, including stroke, TIA, myocardial infarction, or of cardiac obstruction such as syncope, heart failure, or sudden death. The most common presentation is a stroke in 30% of patients. It is also noted to be an incidental finding in one-third of patients.[8] Papillary fibroelastomas do not lead to physical exam finding alterations, although if the PFE is causing sequelae such as obstruction, then the physical exam will identify findings consistent with that complication. The diagnosis of PFE purely on history and physical exam is extremely rare.
Evaluation
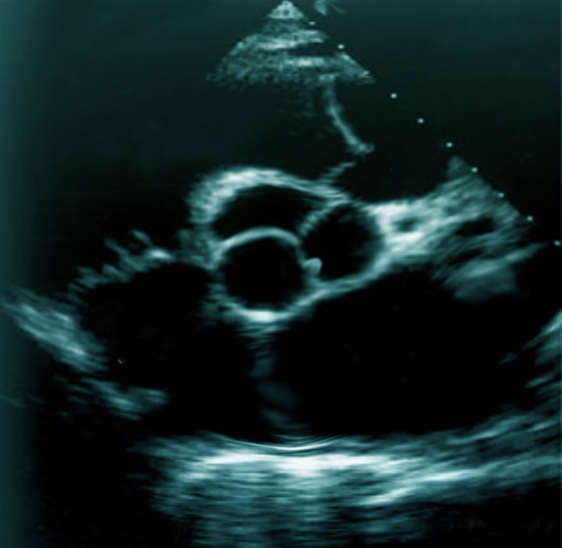
Transthoracic echocardiography (TTE) should be the initial approach as it is the least invasive way of diagnosing papillary fibroelastomas. The sensitivity and specificity of TTE for the detection of PFE greater than 2 mm are 88.9% and 87.8%, respectively. However, if the index of suspicion is high for a cardioembolic source of stroke, a transesophageal echocardiogram (TEE) can be obtained to visualize the intracardiac structures better.[9] The overall sensitivity of PFE less than 2 mm is 61.9% for TTE and 76.6% for TEE. In the study by Tamin et al., 47% of patients had both TTE and TEE done, 27% had TEE alone, and 17% had TTE only.[1] A majority of PFEs are found incidentally at the time of imaging via echocardiography, cardiac surgery, or autopsy.
Papillary fibroelastoma sizes on echocardiography generally range from 2 mm to 40 mm and are usually found to be pedunculated. They have independent mobility with a speckle pattern along the edges of the PFE. PFEs may arise from the aortic or ventricular surface of the valves. In the study by Tamin et al., the lesion occurred in equal proportions on both sides of the valves. Other published literature has reported ambiguous results regarding location, with some studies reporting PFEs more commonly found on the aortic side of the aortic valve leaflets, while other studies demonstrated PFE on the ventricular side of the aortic valve.[10] Cardiac magnetic resonance imaging (MRI) and computed tomography (CT) can potentially offer better spatial resolution than echocardiography. However, these modalities, particularly cardiac MRI, are significantly more expensive and require specialized resources for the performance and evaluation of the study.[11] As with other solid tumors, definitive diagnosis requires pathologic analysis and examination.
Treatment / Management
The two major treatment options include surgery or close monitoring. Generally, surgical excision is the recommended treatment unless there is a contraindication to surgery. The goal of surgical treatment is to excise all visible tumor and reconstruct any resultant intracardiac defects. Papillary fibroelastoma tumors are typically attached to a pedicle and are excisable with precision. Other chambers of the heart are inspected to avoid missing multiple PFEs. If the PFE significantly damages a valve, it may warrant valve repair or replacement at the time of surgery. If the patient is a poor surgical candidate and yet they are symptomatic, then consideration of anticoagulation is reasonable. The rationale for this approach is to prevent embolic complications. However, there are no randomized controlled trials to support this practice. Asymptomatic patients with a PFE size over 9 mm, highly mobile masses, and independent motion are predictors of poor outcomes and, thus, should be considered for surgery.[4]
Differential Diagnosis
The differential diagnosis for a cardiac valvular mass is large, but it can be broken down into categories based on clinical presentation. If a patient is presenting with concerning signs of infection, then a TTE or TEE guided identification of mass is strongly suggestive of endocarditis. If the mass is present in the clinical setting of lupus or inflammatory states, consider non-bacterial or marantic endocarditis. A thrombus, Lambl’s excrescence, or valvular calcifications should merit consideration in the differential. A majority of cardiac masses are secondary to metastatic cancer of the breast, melanoma, hepatocellular, renal, or sarcoma. Primary malignant tumors are rare, but the differential includes angiosarcoma and leiomyosarcoma, which typically do not involve the aortic valve.[11]
Surgical Oncology
Surgery for papillary fibroelastoma excision requires a full cardiopulmonary bypass. Depending on risk factors (i.e., personal and family history), pre-operative coronary evaluation via catheterization may be helpful. If the tumor is on the aortic side of the aortic valve, there may be some concern about possible emboli due to dislodgement induced by the angiography catheter. Traditionally, the heart is approached via median sternotomy. Arterial cannulation is achieved with the ascending aorta. Venous cannulation is achievable via dual-stage through the right atrium or bicaval, depending on the presumed location of the tumor. Bicaval cannulation allows for the most flexibility in case the right atrium must be opened. Cardioplegia can be given antegrade only or both antegrade and retrograde. Aortic cross-clamping with full arrest is necessary. For tumors involving the aortic valve, an aortotomy (transverse or hockey stick) is made in the proximal ascending aorta. Exposure is obtained, and the aortic valve is inspected. The PFE mass is gently excised off the valve while minimizing trauma to the leaflets. All visible tumor mass requires excision; however, sometimes, this will require the removal of part of the leaflet. The LVOT is thoroughly inspected as well, along with the ventricular side of the mitral leaflets. If the aortic valve is competent, then the aortotomy is closed. If the aortic valve is not competent, either primary repair or replacement is necessary. The patient is then de-aired as per routine and weaned off cardiopulmonary bypass. TEE must be performed before decannulation to ensure that there are no other obvious abnormalities seen. Any specimens require a histological examination for pathology. Frozen section is not routinely necessary.
Prognosis
The prognosis of papillary fibroelastoma is generally good following the excision of the tumor. Because of their benign nature, there is a low risk of metastatic spread or recurrence. The latter may occur if there was an incomplete excision of the tumor at the time of initial surgery.
Complications
Complications are based on the risk of embolization. Studies have reported the risk of sudden cardiac death, ventricular fibrillation, myocardial infarction, and stroke. The risks following surgery include those associated with any intracardiac surgery on cardiopulmonary bypass, including emboli, bleeding, arrhythmias, and infection.
Postoperative and Rehabilitation Care
Postoperative care is standard as with other open-heart surgical operations via median sternotomy. Ambulation on postoperative day one is encouraged. Drainage tube removal follows as per institutional routine. Depending on mobility, postoperative cardiac rehabilitation and/or physical therapy are reasonable, particularly in deconditioned or older patients.
Consultations
Upon identification of an intracardiac mass, consultation with cardiac surgery and cardiology is necessary.
Deterrence and Patient Education
It is important to note that while it is the leading primary cardiac tumor, it is a rare tumor with an incidence of less than 0.1%. Typically identified incidentally on transthoracic echocardiogram, papillary fibroelastomas should be evaluated initially by a cardiologist and cardiothoracic surgeon. PFEs do not typically have symptoms unless they embolize and cause arterial occlusion in the organ bed. If PFEs embolize to the coronary arteries, this will lead to myocardial infarction. If it embolizes to the brain, it may cause a stroke. However, there are no clearly identifiable risk factors for the development of PFE.
Enhancing Healthcare Team Outcomes
Diagnosis of papillary fibroelastoma is challenging and often incidentally discovered after an event. For instance, the differential diagnosis for a cerebrovascular accident involves a cardioembolic source of stroke and often evokes reference for left atrial or ventricular thrombus embolization. However, a PFE should also be in the differential diagnosis, as embolization can lead to stroke.
The symptomatic stroke patient may initially present in the care of a neurologist, who may then refer the patient to a cardiologist to determine the cardiac etiology. In some instances, the emergency department physician or an internist may be the first physicians to evaluate symptoms of chest pain and recognize a myocardial infarction and refer the patient urgently under cardiology care. Once the interventional cardiology team determines the cause of the patient's chest pain is not an acute plaque rupture event and rather an embolization event, further workup with imaging studies with TTE and TEE follows. Once the diagnosis of PFE results from typical imaging findings, a cardiothoracic surgeon with experience in removing PFEs should be on the case. No randomized trial data exists on the medical or surgical management outcomes for PFE. The level of evidence is Level 4, as papillary fibroelastomas are a rare occurrence.
In the few cases where surgery is not possible, a cardiology board-certified pharmacist can assist with appropriate anticoagulation therapy. Cardiology specialty nurses can also prove invaluable for follow-up management in both surgical and medical cases by charting the progress of treatment, counseling the patients, answering their questions, and reporting any concerns to the rest of the interprofessional healthcare team. In this interprofessional team paradigm, all disciplines share information about the case, everyone on the team is kept current, and the patient can achieve optimal outcomes. [Level 5]