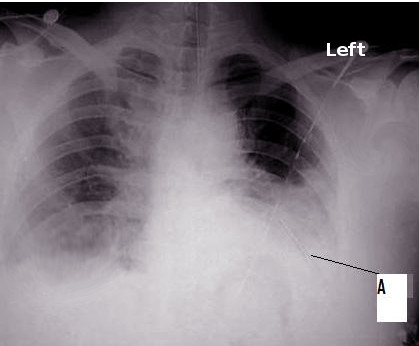
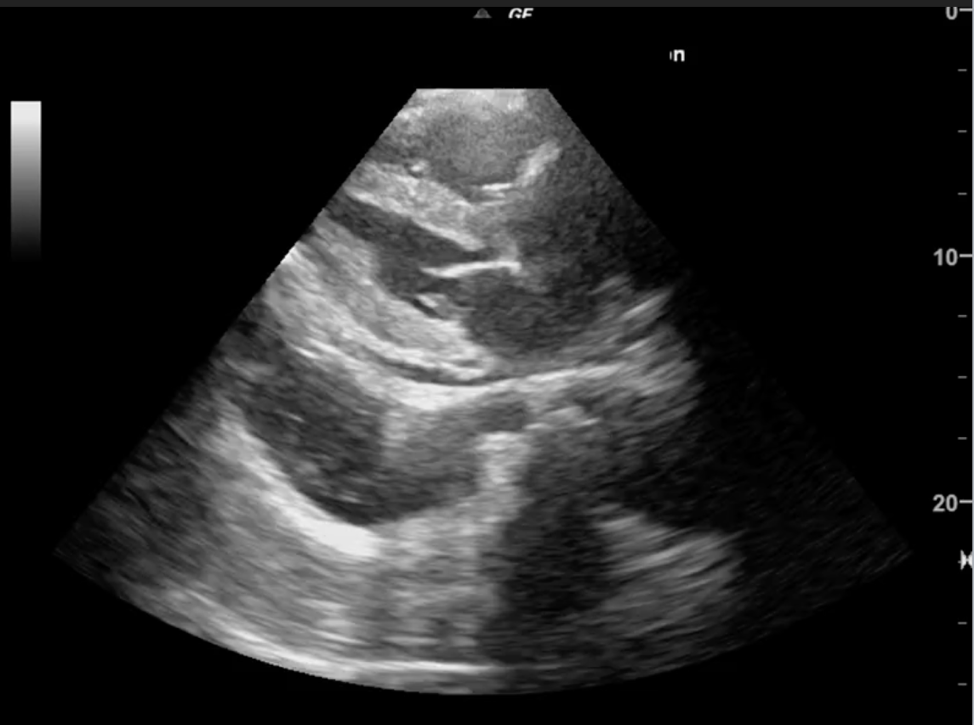
[1]
Brooks A, Davies B, Smethhurst M, Connolly J. Emergency ultrasound in the acute assessment of haemothorax. Emergency medicine journal : EMJ. 2004 Jan:21(1):44-6
[PubMed PMID: 14734374]
[2]
Stengel D, Leisterer J, Ferrada P, Ekkernkamp A, Mutze S, Hoenning A. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. The Cochrane database of systematic reviews. 2018 Dec 12:12(12):CD012669. doi: 10.1002/14651858.CD012669.pub2. Epub 2018 Dec 12
[PubMed PMID: 30548249]
Level 1 (high-level) evidence
[3]
Abboud PA, Kendall J. Emergency department ultrasound for hemothorax after blunt traumatic injury. The Journal of emergency medicine. 2003 Aug:25(2):181-4
[PubMed PMID: 12902006]
[4]
McEwan K, Thompson P. Ultrasound to detect haemothorax after chest injury. Emergency medicine journal : EMJ. 2007 Aug:24(8):581-2
[PubMed PMID: 17652688]
[5]
Staub LJ, Biscaro RRM, Kaszubowski E, Maurici R. Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: A systematic review and meta-analysis. Injury. 2018 Mar:49(3):457-466. doi: 10.1016/j.injury.2018.01.033. Epub 2018 Feb 8
[PubMed PMID: 29433802]
Level 1 (high-level) evidence
[6]
Rahimi-Movaghar V, Yousefifard M, Ghelichkhani P, Baikpour M, Tafakhori A, Asady H, Faridaalaee G, Hosseini M, Safari S. Application of Ultrasonography and Radiography in Detection of Hemothorax; a Systematic Review and Meta-Analysis. Emergency (Tehran, Iran). 2016 Summer:4(3):116-26
[PubMed PMID: 27299139]
Level 1 (high-level) evidence
[7]
Vafaei A, Hatamabadi HR, Heidary K, Alimohammadi H, Tarbiyat M. Diagnostic Accuracy of Ultrasonography and Radiography in Initial Evaluation of Chest Trauma Patients. Emergency (Tehran, Iran). 2016 Winter:4(1):29-33
[PubMed PMID: 26862547]
[8]
Broderick SR. Hemothorax: Etiology, diagnosis, and management. Thoracic surgery clinics. 2013 Feb:23(1):89-96, vi-vii. doi: 10.1016/j.thorsurg.2012.10.003. Epub 2012 Nov 3
[PubMed PMID: 23206720]
[9]
Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respiratory medicine. 2010 Nov:104(11):1583-7. doi: 10.1016/j.rmed.2010.08.006. Epub
[PubMed PMID: 20817498]
[10]
Goodman M, Lewis J, Guitron J, Reed M, Pritts T, Starnes S. Video-assisted thoracoscopic surgery for acute thoracic trauma. Journal of emergencies, trauma, and shock. 2013 Apr:6(2):106-9. doi: 10.4103/0974-2700.110757. Epub
[PubMed PMID: 23723618]
[11]
Özdil A, Kavurmacı Ö, Akçam Tİ, Ergönül AG, Uz İ, Şahutoğlu C, Yüzkan S, Çakan A, Çağırıcı U. A pathology not be overlooked in blunt chest trauma: Analysis of 181 patients with bilateral pneumothorax. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery : TJTES. 2018 Nov:24(6):521-527. doi: 10.5505/tjtes.2018.76435. Epub
[PubMed PMID: 30516250]
[12]
Sirmali M, Türüt H, Topçu S, Gülhan E, Yazici U, Kaya S, Taştepe I. A comprehensive analysis of traumatic rib fractures: morbidity, mortality and management. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2003 Jul:24(1):133-8
[PubMed PMID: 12853057]
[13]
Ota K, Fumimoto S, Iida R, Kataoka T, Ota K, Taniguchi K, Hanaoka N, Takasu A. Massive hemothorax due to two bleeding sources with minor injury mechanism: a case report. Journal of medical case reports. 2018 Oct 7:12(1):291. doi: 10.1186/s13256-018-1813-x. Epub 2018 Oct 7
[PubMed PMID: 30292243]
Level 3 (low-level) evidence
[14]
Morley EJ, Johnson S, Leibner E, Shahid J. Emergency department evaluation and management of blunt chest and lung trauma (Trauma CME). Emergency medicine practice. 2016 Jun:18(6):1-20
[PubMed PMID: 27177417]
[15]
Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Current opinion in pulmonary medicine. 2015 Jul:21(4):393-8. doi: 10.1097/MCP.0000000000000173. Epub
[PubMed PMID: 25978625]
Level 3 (low-level) evidence
[16]
Scott MF, Khodaverdian RA, Shaheen JL, Ney AL, Nygaard RM. Predictors of retained hemothorax after trauma and impact on patient outcomes. European journal of trauma and emergency surgery : official publication of the European Trauma Society. 2017 Apr:43(2):179-184. doi: 10.1007/s00068-015-0604-y. Epub 2015 Nov 30
[PubMed PMID: 26619854]
[19]
Richards JR, McGahan JP. Focused Assessment with Sonography in Trauma (FAST) in 2017: What Radiologists Can Learn. Radiology. 2017 Apr:283(1):30-48. doi: 10.1148/radiol.2017160107. Epub
[PubMed PMID: 28318439]
[20]
Hyacinthe AC, Broux C, Francony G, Genty C, Bouzat P, Jacquot C, Albaladejo P, Ferretti GR, Bosson JL, Payen JF. Diagnostic accuracy of ultrasonography in the acute assessment of common thoracic lesions after trauma. Chest. 2012 May:141(5):1177-1183. doi: 10.1378/chest.11-0208. Epub 2011 Oct 20
[PubMed PMID: 22016490]
[21]
Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheffield, England). 2017 Jun:13(2):100-111. doi: 10.1183/20734735.004717. Epub
[PubMed PMID: 28620429]
[22]
Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015 Jun:147(6):1659-1670. doi: 10.1378/chest.14-1313. Epub
[PubMed PMID: 26033127]
[23]
Zieleskiewicz L, Fresco R, Duclos G, Antonini F, Mathieu C, Medam S, Vigne C, Poirier M, Roche PH, Bouzat P, Kerbaul F, Scemama U, Bège T, Thomas PA, Flecher X, Hammad E, Leone M. Integrating extended focused assessment with sonography for trauma (eFAST) in the initial assessment of severe trauma: Impact on the management of 756 patients. Injury. 2018 Oct:49(10):1774-1780. doi: 10.1016/j.injury.2018.07.002. Epub 2018 Jul 8
[PubMed PMID: 30017184]
[24]
Soni NJ, Franco R, Velez MI, Schnobrich D, Dancel R, Restrepo MI, Mayo PH. Ultrasound in the diagnosis and management of pleural effusions. Journal of hospital medicine. 2015 Dec:10(12):811-6. doi: 10.1002/jhm.2434. Epub 2015 Jul 28
[PubMed PMID: 26218493]
[25]
Ibitoye BO, Idowu BM, Ogunrombi AB, Afolabi BI. Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography (Seoul, Korea). 2018 Jul:37(3):254-260. doi: 10.14366/usg.17050. Epub 2017 Oct 18
[PubMed PMID: 29228764]
[26]
Dennis BM, Gondek SP, Guyer RA, Hamblin SE, Gunter OL, Guillamondegui OD. Use of an evidence-based algorithm for patients with traumatic hemothorax reduces need for additional interventions. The journal of trauma and acute care surgery. 2017 Apr:82(4):728-732. doi: 10.1097/TA.0000000000001370. Epub
[PubMed PMID: 28099387]
[27]
Gleeson T, Blehar D. Point-of-Care Ultrasound in Trauma. Seminars in ultrasound, CT, and MR. 2018 Aug:39(4):374-383. doi: 10.1053/j.sult.2018.03.007. Epub 2018 Mar 29
[PubMed PMID: 30070230]
[28]
Tian Y, Zheng W, Zha N, Wang Y, Huang S, Guo Z. Thoracoscopic decortication for the management of trapped lung caused by 14-year pneumothorax: A case report. Thoracic cancer. 2018 Aug:9(8):1074-1077. doi: 10.1111/1759-7714.12770. Epub 2018 May 26
[PubMed PMID: 29802756]
Level 3 (low-level) evidence
[29]
Bunn T, Singleton M, Chen IC. Use of multiple data sources to identify specific drugs and other factors associated with drug and alcohol screening of fatally injured motor vehicle drivers. Accident; analysis and prevention. 2019 Jan:122():287-294. doi: 10.1016/j.aap.2018.10.012. Epub 2018 Nov 2
[PubMed PMID: 30396030]
[30]
Zhu M, Li Y, Wang Y. Design and experiment verification of a novel analysis framework for recognition of driver injury patterns: From a multi-class classification perspective. Accident; analysis and prevention. 2018 Nov:120():152-164. doi: 10.1016/j.aap.2018.08.011. Epub 2018 Aug 20
[PubMed PMID: 30138770]
Level 3 (low-level) evidence
[31]
Morgan E. Driving Dilemmas: A Guide to Driving Assessment in Primary Care. Clinics in geriatric medicine. 2018 Feb:34(1):107-115. doi: 10.1016/j.cger.2017.09.006. Epub
[PubMed PMID: 29129210]
[32]
Ecola L, Popper SW, Silberglitt R, Fraade-Blanar L. The Road to Zero: A Vision for Achieving Zero Roadway Deaths by 2050. Rand health quarterly. 2018 Oct:8(2):11
[PubMed PMID: 30323994]
[33]
Han GM, Newmyer A, Qu M. Seatbelt use to save money: Impact on hospital costs of occupants who are involved in motor vehicle crashes. International emergency nursing. 2017 Mar:31():2-8. doi: 10.1016/j.ienj.2016.04.004. Epub 2016 May 10
[PubMed PMID: 27177737]
[34]
Chung MH, Hsiao CY, Nian NS, Chen YC, Wang CY, Wen YS, Shih HC, Yen DH. The Benefit of Ultrasound in Deciding Between Tube Thoracostomy and Observative Management in Hemothorax Resulting from Blunt Chest Trauma. World journal of surgery. 2018 Jul:42(7):2054-2060. doi: 10.1007/s00268-017-4417-5. Epub
[PubMed PMID: 29305713]
[35]
Carver DA, Bressan AK, Schieman C, Grondin SC, Kirkpatrick AW, Lall R, McBeth PB, Dunham MB, Ball CG. Management of haemothoraces in blunt thoracic trauma: study protocol for a randomised controlled trial. BMJ open. 2018 Mar 3:8(3):e020378. doi: 10.1136/bmjopen-2017-020378. Epub 2018 Mar 3
[PubMed PMID: 29502092]
Level 1 (high-level) evidence