Introduction
The spleen is a site of a variety of pathologic processes, including congenital, infectious, traumatic, vascular, and neoplastic, among others. Multiple modalities are useful to image the spleen.

The spleen is a site of a variety of pathologic processes, including congenital, infectious, traumatic, vascular, and neoplastic, among others. Multiple modalities are useful to image the spleen.
The spleen is an intraperitoneal organ located in the left upper quadrant of the abdomen. The spleen is maintained in its normal anatomic position by two ligaments, the gastrosplenic and splenorenal ligaments. Without these ligamentous supports, the spleen can move around the abdomen, a condition known as wandering spleen. A wandering spleen is at increased risk for torsion.[1]
The normal adult spleen generally measures no greater than 12 cm x 7 cm x 4 cm. Splenomegaly is loosely defined as a spleen larger than 13 to 14 cm in the craniocaudal dimension, although the splenic index is the most accurate measure of splenic volume. This metric is a volumetric index calculated as a product of length x width x height. Various pathologic conditions can lead to splenomegaly, such as portal hypertension and lymphoma.[2]
Normal anatomic variants are commonly encountered when imaging the spleen. Splenic clefts are invaginations of the splenic capsule, which create septations in the splenic parenchyma. Splenules are round masses of splenic tissue measuring up to several centimeters in size that are separate from but regional to the spleen, most commonly at the splenic hilum (see Image. Splenule). It is essential to recognize these anatomic variants so as not to mistake them for pathologies, such as a splenic laceration or enlarged lymph node.[1][3]
The splenic artery conventionally arises from the celiac axis and travels along the superior aspect of the pancreas to reach the splenic hilum. The splenic vein originates from the splenic hilum and courses along the posterior pancreas, joining the superior mesenteric vein to create the main portal vein. In the setting of portal hypertension or splenic vein occlusion, there are numerous collaterals for splenic venous outflow, including venous connections between the short gastric and coronary vein as well as left and right gastroepiploic veins. A splenorenal shunt is an abnormal connection between the splenic vein and the left renal vein. Splenic vein thrombosis occurs in about 20% of patients with chronic pancreatitis.[4]
The spleen is composed of red and white pulp. The white pulp is the site of lymphoid tissue, while the red pulp functions to filter the blood, removing old or damaged red blood cells and pathogens.[1]
The plain abdominal radiograph has limited value for evaluating the spleen. It is possible to glean some information from these studies, including the presence or absence of calcifications and the size of the spleen.[2] Vascular calcifications are visible in some cases, as in a tortuous calcified splenic artery or rim calcified splenic artery aneurysm.[4]
Computed tomography (CT) is one of the major modalities used for evaluating the spleen along with ultrasound (US). The normal spleen measures approximately 40 to 60 Hounsfield units (HU) on unenhanced CT, about 10 HU less than that of the liver. On unenhanced CT, the examiner can easily appreciate splenic calcifications. Single or multiple small rounded calcifications usually represent calcified granulomas.[5] Peripheral calcifications can occur after splenic infarct. Curvilinear calcifications along the capsule are visible with old resolved hematomas.
On an enhanced CT, the spleen demonstrates a serpentine, cordlike, irregular enhancement pattern in the arterial phase (approximately 30 seconds after injection of intravenous contrast) due to the presence of an open circulatory system in the red pulp. On portal venous phase imaging (60 seconds after injection of intravenous contrast), the spleen enhances homogenously since the contrast opacifies both the red and white pulp.[4]
The spleen is the most frequently injured abdominal organ. Contrast-enhanced CT is necessary for a complete evaluation of splenic trauma. Nonenhanced studies are suboptimal and may miss subtle lacerations. Traumatic findings include laceration, subcapsular or intraparenchymal hematoma, vascular injury, shattered spleen, and active hemorrhage. The American Association for the Surgery of Trauma (AAST) is the most widely used grading system for splenic trauma; imaging features are the basis of the grading system. Splenic trauma is usually associated with overlying rib fractures.[2]
A splenic infarct characteristically presents on CT as a peripheral wedge-shaped hypodense lesion (see Image. Splenic Infarcts). The causes of splenic infarcts include cardioembolic events, vasculitis, hematologic phenomena, splenic vein thrombosis, pancreatitis, and iatrogenic causes. Chronic infarct results in notching of the normally smooth splenic contour and peripheral calcifications. In patients with sickle cell disease, numerous chronic infarcts result in autosplenectomy, seen on CT as a shrunken spleen with diffuse calcification.[5]
True cysts are congenital epithelial lined fluid-filled structures. On CT, they appear as rounded, non-enhancing, hypodense lesions with internal attenuation similar to that of water (0 HU). Histologically, they have thin walls, but the walls are normally beyond the resolution of CT and, therefore, imperceptible. False cysts are not lined by epithelium and result from prior trauma, infection, or infarct. On CT, they often appear similar to true cysts. Distinguishing features, when present, including higher internal density due to the presence of debris or blood products and peripheral calcifications. Pancreatic pseudocysts can take on an intrasplenic location.[5][6]
A pyogenic abscess occurs most commonly by the hematogenous spread of bacteria, especially in immunocompromised patients. These lesions display central hypodensity like cysts, but classically with have ill-defined, irregular, thick, enhancing walls. The additional imaging feature of anti-dependent gas in the collection is diagnostic but not always present [5].
The most frequently encountered primary neoplasm of the spleen is a hemangioma, which is a benign tumor of vascular origin lined with endothelium and filled with red blood cells. On unenhanced CT, these lesions may look like cysts. The capillary form typically presents as a well defined homogenously enhancing nodule.[7] Whereas hemangiomas in the liver classically display peripheral nodular enhancement with centripetal filling on later phase imaging, cavernous hemangiomas in the spleen do not always show this pattern and commonly show heterogeneous enhancement given their cystic and solid components (see Image. Heterogeneous Splenic Enhancement). Hemangiomas can contain calcifications.[8] The spleen is involved in about one-third of patients with lymphoma, but primary lymphoma of the spleen is rare. The CT appearance of the spleen in this disease process is variable. The lymphomatous spleen may appear normal or enlarged on CT. Multiple hypodense, non-enhancing splenic lesions are a nonspecific finding seen in lymphoma, sarcoid, fungal microabscesses, and opportunistic infections.[2][5][9]
Angiosarcoma is exceedingly rare; however, it is the most common primary malignant vascular tumor of the spleen. It is highly aggressive and portrays a poor prognosis, usually with metastatic disease on presentation. The imaging characteristics are varied. On CT, multiple hypervascular, heterogeneous masses in the spleen with necrosis, hemorrhage, and distant metastases would be compatible with this malignancy.[10]
CT and US are the primary modalities used for imaging the spleen. MRI can be helpful in select cases.
The normal signal of the adult spleen on MRI is hyperintense on T2-weighted imaging and hypointense on T1-weighted imaging relative to the liver.[11] Deviations from this pattern can reflect disease processes such as hemochromatosis and hemosiderosis. The spleen is high in signal on diffusion-weighted imaging. In the neonate, the spleen is hypointense relative to the liver on T1- and T2-weighted imaging.[12]
Hemangiomas are identified on MR as hypointense on T1-weight imaging and hyperintense on T2-weighted imaging. Contrast-enhanced imaging patterns will be similar to contrast-enhanced CT and can show peripheral enhancement with centripetal filling on later phase imaging, although not all hemangiomas show this pattern.[11] A lymphangioma is a benign endothelial-lined lesion filled with simple lymphatic fluid; sometimes, the fluid can be proteinaceous. On MRI, they are T2 hyperintense, T1 hypointense, non-enhancing, multiloculated cystic structures, which usually appear in the periphery of the spleen.[8] A simple cyst will appear hyperintense on T2, owing to the presence of water. Lack of complexity, including thin walls and minimal septations, confirms the diagnosis.[6][11]
Different types of lymphoma can produce various manifestations in the spleen, including splenomegaly, diffuse miliary splenic infiltration, large focal mass, etc.[13] Lymphomatous infiltrates are usually hypo-enhancing as compared to the rest of the splenic parenchyma. MRI can sometimes be misleading in identifying focal infiltrating lymphoma due to little tissue contrast between normal splenic parenchyma and lymphomatous tissue on some sequences.[5]
On ultrasound, the normal spleen has a uniform echogenicity and is hypoechoic relative to the cortex of the nearby kidney (see Image. Ultrasound of the Spleen).[2]
A simple cyst will appear similar to cysts elsewhere in the body. Characteristic features of cysts on ultrasound include round shape, anechoic echogenicity, and pencil-thin walls. Posterior acoustic enhancement and absence of internal Doppler flow are additional features.[2][6] False cysts are more likely to have internal echoes due to internal debris and peripheral echogenic foci representing peripheral calcifications. Abscesses appear as hypoechoic lesions with irregular walls and sometimes contain echogenic gas. Splenic infarcts appear as a hypoechoic peripheral wedge-shaped area on ultrasound. Chronic infarcts are hyperechoic due to scarring.[2] On the ultrasound exam, lymphangiomas appear as thin-walled cystic lesions with septations and internal debris. Color Doppler will show flow along the cyst walls.[10]
Tc-99m sulfur colloid or Tc-99m heat-damaged red blood cells are radiotracers used to identify splenic tissue. In both cases, the radiotracer is injected intravenously and taken up by the spleen, after which a gamma camera is used to acquire images. A major indication for the study is detecting splenic tissue in the setting of splenosis, which is heterotopic implantation of splenic tissue occurring in cases of splenic trauma or splenectomy. Other indications include differentiating a mass closely related to the hemidiaphragm, as a diagnostic adjunct in spleno-gonadal fusion, asplenia, and polysplenia syndromes, and identifying a wandering spleen.[1][14]
Angiography is generally performed when an intervention is planned, such as splenic artery pseudoaneurysm embolization or splenic artery embolization in the setting of splenic trauma (see Image. Digital Subtraction Angiography). Trauma patients are candidates for splenic artery embolization if they are hemodynamically stable with AAST grade 3 or higher injury, active contrast extravasation on CT, or splenic vascular injury.[15]
Many splenic lesions have overlapping imaging features, making patient history, physical exam, and laboratory values helpful in narrowing the differential diagnosis. CT is a major imaging modality for evaluating splenic pathology. Splenic trauma is a unique scenario where CT is essential in diagnosis, and management requires close communication between the emergency department clinician, surgeon, radiologist, and interventionalist. More often, splenic abnormalities are noted incidentally on CT performed for another indication. The clinician's knowledge of normal and abnormal findings will prevent unnecessary intervention or work-up for incidental or benign lesions. Systemic disorders that involve the spleen are often more clinically significant than solitary splenic lesions.
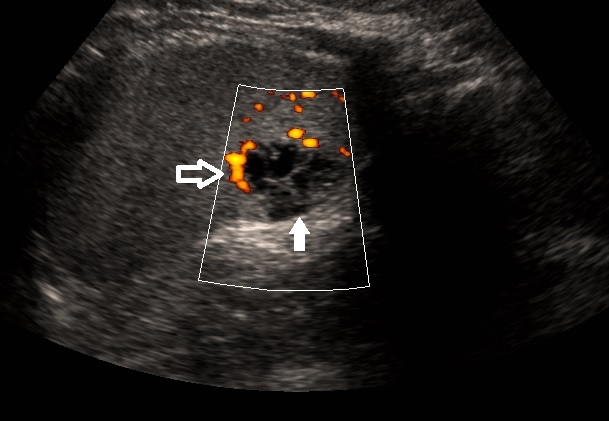
Ultrasound of the Spleen. Ultrasound of the spleen in sagittal plane demonstrates a multilocular cystic structure in the periphery of the spleen (solid white arrow). Power doppler shows flow along the wall of the cystic lesion (open white arrow). Findings are compatible with lymphangioma.
Contributed by W Coffey, MD
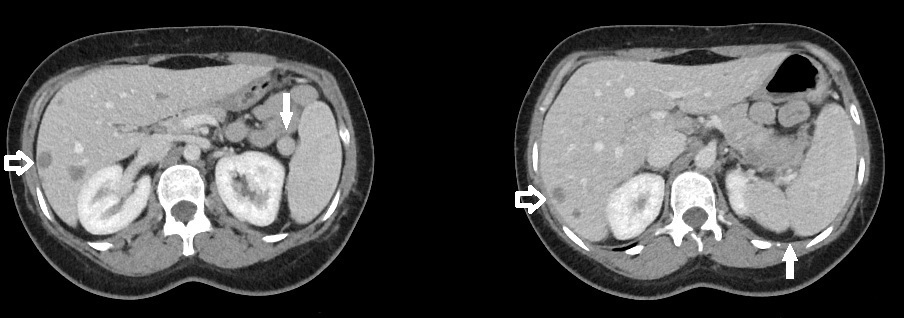
Splenule. Axial slice from a CT of the upper abdomen (left) demonstrates a small mass at the splenic hilum with similar attenuation to the nearby spleen consistent with a splenule (solid arrow). A more superior axial slice from the same patient (right) shows a splenic cleft (solid arrow). Also, note the hypodense liver metastases (open arrows).
Contributed by W Coffey, MD
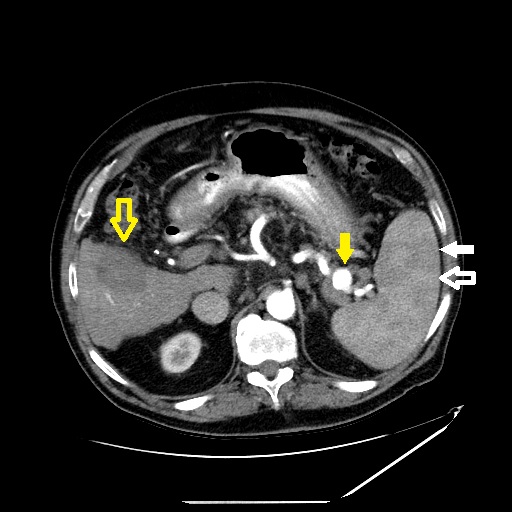
Heterogeneous Splenic Enhancement. Axial slice of a contrast-enhanced CT of the upper abdomen in the arterial phase shows heterogeneous splenic enhancement. The red pulp (open white arrow) enhances earlier than the white pulp (solid white arrow) due to the variable circulatory systems within the spleen. Also, note the splenic artery aneurysm (solid yellow arrow) and hypodense liver lesion compatible with the treatment cavity from recent radiofrequency ablation (open yellow arrow).
Contributed by W Coffey, MD
Freeman JL, Jafri SZ, Roberts JL, Mezwa DG, Shirkhoda A. CT of congenital and acquired abnormalities of the spleen. Radiographics : a review publication of the Radiological Society of North America, Inc. 1993 May:13(3):597-610 [PubMed PMID: 8316667]
Robertson F, Leander P, Ekberg O. Radiology of the spleen. European radiology. 2001:11(1):80-95 [PubMed PMID: 11194923]
Yildiz AE, Ariyurek MO, Karcaaltincaba M. Splenic anomalies of shape, size, and location: pictorial essay. TheScientificWorldJournal. 2013:2013():321810. doi: 10.1155/2013/321810. Epub 2013 Apr 21 [PubMed PMID: 23710135]
Uy PPD, Francisco DM, Trivedi A, O'Loughlin M, Wu GY. Vascular Diseases of the Spleen: A Review. Journal of clinical and translational hepatology. 2017 Jun 28:5(2):152-164. doi: 10.14218/JCTH.2016.00062. Epub 2017 Mar 24 [PubMed PMID: 28660153]
Rabushka LS, Kawashima A, Fishman EK. Imaging of the spleen: CT with supplemental MR examination. Radiographics : a review publication of the Radiological Society of North America, Inc. 1994 Mar:14(2):307-32 [PubMed PMID: 8190956]
Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 1996 Jan:16(1):107-29 [PubMed PMID: 10946694]
Ferrozzi F, Bova D, Draghi F, Garlaschi G. CT findings in primary vascular tumors of the spleen. AJR. American journal of roentgenology. 1996 May:166(5):1097-101 [PubMed PMID: 8615251]
Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Jul-Aug:24(4):1137-63 [PubMed PMID: 15256634]
Warshauer DM, Hall HL. Solitary splenic lesions. Seminars in ultrasound, CT, and MR. 2006 Oct:27(5):370-88 [PubMed PMID: 17048453]
Kaza RK, Azar S, Al-Hawary MM, Francis IR. Primary and secondary neoplasms of the spleen. Cancer imaging : the official publication of the International Cancer Imaging Society. 2010 Aug 13:10(1):173-82. doi: 10.1102/1470-7330.2010.0026. Epub 2010 Aug 13 [PubMed PMID: 20713317]
Ito K, Mitchell DG, Honjo K, Fujita T, Uchisako H, Matsumoto T, Matsunaga N, Honma Y, Yamakawa K. MR imaging of acquired abnormalities of the spleen. AJR. American journal of roentgenology. 1997 Mar:168(3):697-702 [PubMed PMID: 9057518]
Paterson A, Frush DP, Donnelly LF, Foss JN, O'Hara SM, Bisset GS 3rd. A pattern-oriented approach to splenic imaging in infants and children. Radiographics : a review publication of the Radiological Society of North America, Inc. 1999 Nov-Dec:19(6):1465-85 [PubMed PMID: 10555669]
Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N, Jagannathan JP. Spleen in haematological malignancies: spectrum of imaging findings. The British journal of radiology. 2012 Jan:85(1009):81-92. doi: 10.1259/bjr/31542964. Epub 2011 Nov 17 [PubMed PMID: 22096219]
Sty JR, Conway JJ. The spleen: development and functional evaluation. Seminars in nuclear medicine. 1985 Jul:15(3):276-98 [PubMed PMID: 3898381]
Imbrogno BF, Ray CE. Splenic artery embolization in blunt trauma. Seminars in interventional radiology. 2012 Jun:29(2):147-9. doi: 10.1055/s-0032-1312577. Epub [PubMed PMID: 23729986]