Continuing Education Activity
Anagen effluvium is a form of nonscarring alopecia commonly associated with chemotherapy. In this disorder, affected anagen hairs suffer a toxic or inflammatory insult, resulting in fracture of the hair shaft. Anagen effluvium is often referred to as chemotherapy-induced alopecia, as it can be triggered by antimetabolites, alkylating agents, and mitotic inhibitors administered as chemotherapeutic therapy. Shedding usually takes place within 14 days of administration of the offending drug, however, in many instances it is reversible, with hair regrowth growth upon discontinuation of the offending agent. This activity examines when this condition should be considered on differential diagnosis and how to properly evaluate for it. This activity highlights the role of the interprofessional team in caring for patients with this condition.
Objectives:
- Identify the etiology of anagen effluvium.
- Describe the history and physical exam findings typically seen in patients with anagen effluvium.
- Outline the management options available to patients with anagen effluvium.
- Explain the modalities to improve coordination among interprofessional team members in order to improve outcomes for patients affected by anagen effluvium.
Introduction
Anagen effluvium is a form of nonscarring alopecia commonly associated with chemotherapy. In this disorder, affected anagen hairs suffer a toxic or inflammatory insult, resulting in fracture of the hair shaft.[1][2][3]
Anagen effluvium is often referred to as chemotherapy-induced alopecia, as it can be triggered by antimetabolites, alkylating agents, and mitotic inhibitors administered as chemotherapeutic therapy. Shedding usually takes place within 14 days of administration of the offending drug, however, in many instances it is reversible, with hair regrowth occurring upon discontinuation of the offending agent. The hair shaft is commonly damaged, and tapered fractures of anagen hairs can be appreciated on trichoscopy.
An understanding of the cyclical phases of hair growth and anatomy of the hair follicle is essential to understanding anagen effluvium. There are roughly 100,000 hairs on the scalp; any given hair is constantly cycling between three stages: anagen, catagen, and telogen. Anagen is a growth phase, lasting between 2 and 6 years with an average of 3 years. Approximately 90% of hairs taken from a normal scalp are anagen hairs. This is a period of epithelial proliferation, in which bulb matrix cells undergo mitosis and proliferation to form the hair shaft. Severe insult to the hair bulb or hair matrix in the form of medications, toxin exposure, or inflammation that causes a cessation of this mitotic activity can cause damage to the hair shaft resulting in breakage, and if the bulb is affected, complete hair loss. Catagen is a transitional phase between anagen and telogen, and in this phase, all growth ceases. Less than 1% of scalp hairs are in catagen at any given time. Telogen is a resting phase, lasting approximately 3 to 5 months, immediately before the hair falls out (teloptosis). Telogen effluvium, a separate entity, occurs when anagen hairs are prematurely shifted into the telogen phase and is triggered by medications, physical or psychological stressors, hospitalization, and pregnancy, among other causes. Kenogen is the lag phase between loss of telogen hair and the growth of new hair.[4][5][6]
Etiology
Several chemotherapy antimetabolites, alkylating agents, and mitotic inhibitors have been implicated in the pathogenesis of anagen effluvium. Doxorubicin, the nitrosoureas, and cyclophosphamide have been implicated too. Several other drugs such as isoniazid (INH), levodopa, colchicine, cyclosporine, and toxic heavy metals such as thallium, mercury, boron, bismuth, copper, and cadmium have also been implicated in triggering anagen effluvium. Anagen effluvium can also be seen in inflammatory disorders such as alopecia areata, and syphilis secondary to inflammatory insult to the hair bulb. Pemphigus vulgaris can also cause anagen effluvium, as desmosomal proteins are expressed in the epithelium of the hair follicle. Anagen effluvium has also been described following severe protein-energy malnutrition states (e.g., Kwashiorkor). Radiation has also been shown to cause both reversible and permanent alopecia. Permanent destruction of the hair follicle occurs when hair follicle stem cells are damaged, and this usually occurs with greater than 30 Gy of deep x-rays.[7][8][9]
Anagen effluvium has been described in a 2-year old male with severe hypotension and hypoxia requiring extracorporeal membrane oxygenation (ECMO). Anagen effluvium is most often secondary to chemotherapy, but it has also been associated with radiotherapy, heavy metal poisoning, protein malnutrition, or systemic diseases. Any of these factors may cause abrupt cessation of mitosis in the hair matrix. [7]
Epidemiology
Anagen effluvium has no gender or regional predilection. It is equally prevalent among men and women across the world.
Histopathology
In anagen effluvium, a punch biopsy of the scalp will exhibit a normal anagen-to-telogen ratio, which is less than 15% telogen hair follicles.
History and Physical
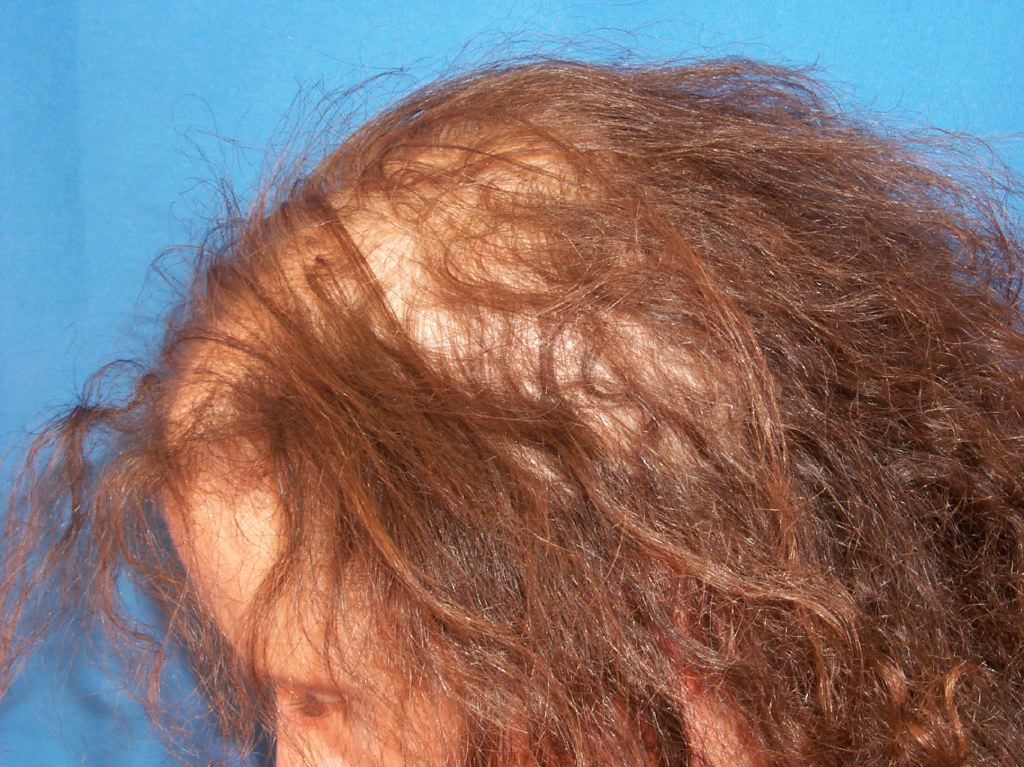
Once chemotherapy has been initiated, anagen effluvium presents within days to a few weeks. The severity of hair loss differs between patients, but it is not unusual for a patient to have complete hair loss within 2 to 3 months of beginning chemotherapy. On physical exam, the patient’s scalp will not display signs of cicatricial alopecia (anagen effluvium is non-cicatricial, and therefore the physician should not be able to appreciate any signs suggestive of an active inflammatory scarring process such as erythema, scaling, or pigmentation). It should be noted that there may be an overlap syndrome of telogen effluvium and anagen effluvium, as the onset of chemotherapy is a significant stressor on the patient.
The hallmark physical exam finding in anagen effluvium is a tapered fracture of the hair shaft. It is possible to identify anagen and telogen hairs with the naked eye alone. However, it can be helpful to observe hair microscopically. Anagen hairs will demonstrate full pigment with roots covered with inner and outer root sheaths, as opposed to telogen hairs that possess club-shaped roots, no inner or outer root sheaths, and depigmentation of the proximal part of the shaft.
Evaluation
A biopsy is rarely necessary, as a diagnosis can usually be made on history and physical exam findings alone. If a biopsy is requested or necessary for diagnosis, it can help exclude telogen effluvium. In anagen effluvium, histopathologic evaluation of a punch biopsy of the scalp will exhibit a normal anagen-to-telogen ratio, which is less than 15% telogen hair follicles. If greater than 15% of the hair follicles are in the telogen phase, this more supports a diagnosis of telogen effluvium.
Treatment / Management
The management of anagen effluvium should be aimed at limiting the amount of time the patient suffers from alopecia. To date, several agents have been studied; unfortunately, no treatment appears to be effective in preventing or stopping the hair loss. Although the results have not been impressive in stopping or preventing hair loss, it has been postulated that topical minoxidil is effective in reducing the period of baldness by an average of fifty days. Several studies have described limiting drug delivery to the scalp by using a scalp tourniquet during chemotherapy. It should be noted, however, that if scalp or brain metastases are a possibility, this method should not be used to allow penetration of the chemotherapeutic agent. Another method that has shown success is inducing scalp hypothermia to a scalp temperature of fewer than 24 degrees Celsius during chemotherapy with daunorubicin, doxorubicin, paclitaxel, vincristine, vinblastine, mechlorethamine, actinomycin D, and epirubicin.[10]
Understandably, hair loss associated with this disorder can be emotionally and psychologically distressing to the patient. Because a pharmacologic agent successful in treating and preventing anagen effluvium has not been found, patient education and aesthetic advice on managing hair loss are fundamental to managing androgen effluvium. Expectations should be managed so that patients understand the unfortunate inevitability of the disorder; however, they should also be assured that most cases of anagen effluvium are reversible and they will grow hair once chemotherapy is ceased. Patients should be instructed to avoid chemical trauma to the hair. This includes hot appliances, bleach, or color treatments in the time leading up to and during chemotherapy. If possible, patients should be given resources to obtaining hairpieces or protective scarves before hair loss and educated on the benefits such garments offer, including cold protection in addition to the aesthetic component.
Differential Diagnosis
The differential diagnosis for anagen effluvium includes other nonscarring alopecias such as telogen effluvium, trichotillomania, and androgenetic alopecia. These entities can be distinguished by history, hair pull test, and trichoscopy. A thorough review of systems should be completed to exclude other causes of hair loss such as nutritional deficiencies, metabolic and endocrine disorders, and infections.
Enhancing Healthcare Team Outcomes
Anagen effluvium is a form of nonscarring alopecia commonly associated with chemotherapy. In this disorder, affected anagen hairs suffer a toxic or inflammatory insult, resulting in fracture of the hair shaft. The healthcare provider and nurse practitioner who encounter such patients should refer these patients to a dermatologist. Shedding usually takes place within 14 days of administration of the offending drug, however, in many instances it is reversible, with hair regrowth occurring upon discontinuation of the offending agent. In anagen effluvium, the hair shaft is commonly damaged, and tapered fractures of anagen hairs can be appreciated on trichoscopy. The prognosis for a patient with anagen effluvium is guarded. While some women may get hair restoration once the chemotherapy is completed, it often takes months or years before full recovery is possible. In a few women, hair thinning may persist without complete recovery.