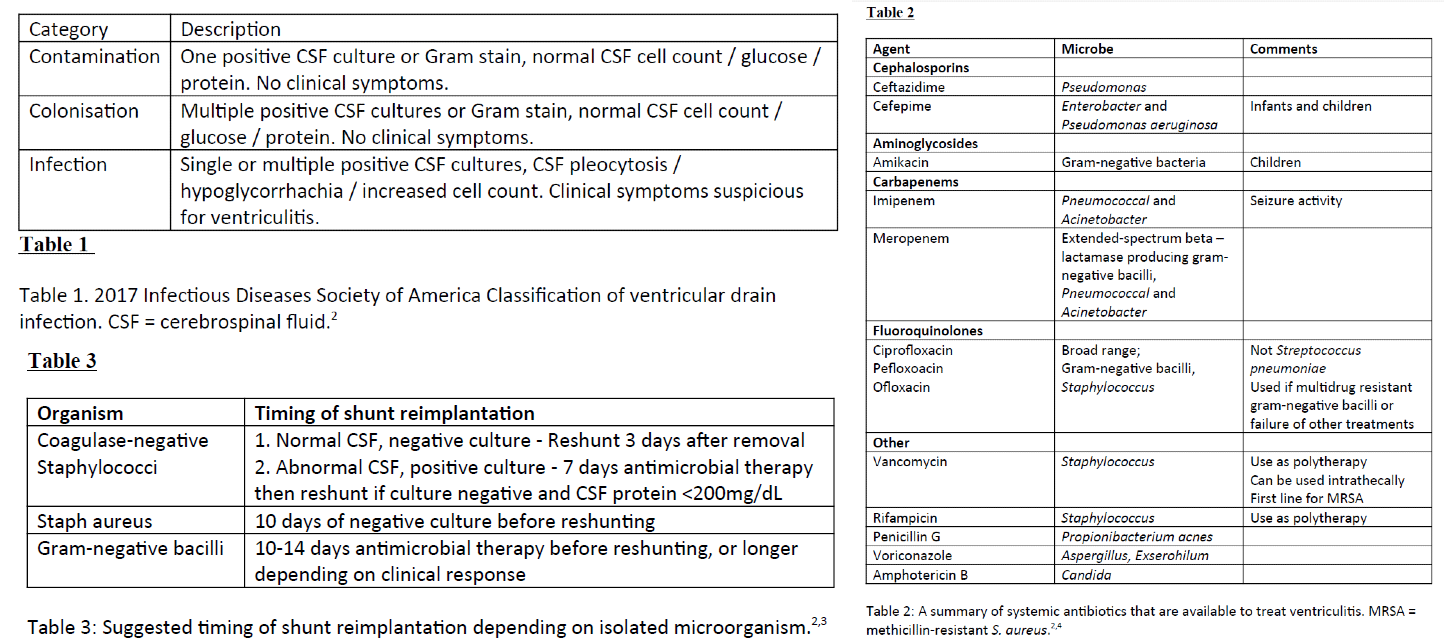
[1]
Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. AJNR. American journal of neuroradiology. 2001 Sep:22(8):1510-6
[PubMed PMID: 11559498]
[2]
Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR. 2017 Infectious Diseases Society of America's Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Mar 15:64(6):e34-e65. doi: 10.1093/cid/ciw861. Epub
[PubMed PMID: 28203777]
Level 1 (high-level) evidence
[3]
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004 Nov 1:39(9):1267-84
[PubMed PMID: 15494903]
Level 1 (high-level) evidence
[4]
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2008 Feb:62 Suppl 2():688-700. doi: 10.1227/01.neu.0000316273.35833.7c. Epub
[PubMed PMID: 18596436]
[5]
Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, Kumar Y. Neonatal cranial sonography: ultrasound findings in neonatal meningitis-a pictorial review. Quantitative imaging in medicine and surgery. 2017 Feb:7(1):123-131. doi: 10.21037/qims.2017.02.01. Epub
[PubMed PMID: 28275563]
[6]
Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, Swartz MN. Acute bacterial meningitis in adults. A review of 493 episodes. The New England journal of medicine. 1993 Jan 7:328(1):21-8
[PubMed PMID: 8416268]
[7]
Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: improved technique can reduce infection rates. British journal of neurosurgery. 2011 Oct:25(5):632-5. doi: 10.3109/02688697.2011.578770. Epub
[PubMed PMID: 21848440]
[8]
Lyke KE, Obasanjo OO, Williams MA, O'Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001 Dec 15:33(12):2028-33
[PubMed PMID: 11712094]
[9]
Humphreys H, Jenks P, Wilson J, Weston V, Bayston R, Waterhouse C, Moore A, Healthcare Infection Society Working Party on Neurosurgical Infections. Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. The Journal of hospital infection. 2017 Feb:95(2):154-160. doi: 10.1016/j.jhin.2016.09.008. Epub 2016 Sep 17
[PubMed PMID: 27756489]
Level 3 (low-level) evidence
[10]
Martin RM, Zimmermann LL, Huynh M, Polage CR. Diagnostic Approach to Health Care- and Device-Associated Central Nervous System Infections. Journal of clinical microbiology. 2018 Nov:56(11):. doi: 10.1128/JCM.00861-18. Epub 2018 Oct 25
[PubMed PMID: 30135235]
[11]
Nilsson A, Uvelius E, Cederberg D, Kronvall E. Silver-Coated Ventriculostomy Catheters Do Not Reduce Rates of Clinically Diagnosed Ventriculitis. World neurosurgery. 2018 Sep:117():e411-e416. doi: 10.1016/j.wneu.2018.06.045. Epub 2018 Jun 18
[PubMed PMID: 29920387]
[12]
Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, Narayan RK. Ventriculostomy-related infections. A prospective epidemiologic study. The New England journal of medicine. 1984 Mar 1:310(9):553-9
[PubMed PMID: 6694707]
[13]
Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. Journal of neurology. 2008 Nov:255(11):1617-24. doi: 10.1007/s00415-008-0059-8. Epub 2008 Dec 8
[PubMed PMID: 19156484]
[14]
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta neurochirurgica. 2008 Mar:150(3):209-14; discussion 214. doi: 10.1007/s00701-007-1458-9. Epub 2008 Feb 19
[PubMed PMID: 18278575]
[15]
Breeze RE, McComb JG, Hyman S, Gilles FH. CSF production in acute ventriculitis. Journal of neurosurgery. 1989 Apr:70(4):619-22
[PubMed PMID: 2647919]
[16]
Firth G, Rees J, McKeran RO. The value of the measurement of cerebrospinal fluid levels of lysozyme in the diagnosis of neurological disease. Journal of neurology, neurosurgery, and psychiatry. 1985 Jul:48(7):709-12
[PubMed PMID: 4031917]
[17]
Schroeder S, Stuerenburg HJ, Escherich F, Pfeiffer G. Lysozyme in ventriculitis: a marker for diagnosis and disease progression. Journal of neurology, neurosurgery, and psychiatry. 2000 Nov:69(5):696-7
[PubMed PMID: 11032636]
[18]
Sakushima K, Hayashino Y, Kawaguchi T, Jackson JL, Fukuhara S. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. The Journal of infection. 2011 Apr:62(4):255-62. doi: 10.1016/j.jinf.2011.02.010. Epub 2011 Mar 5
[PubMed PMID: 21382412]
Level 1 (high-level) evidence
[19]
Williams TA, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. Journal of neurosurgery. 2011 Nov:115(5):1040-6. doi: 10.3171/2011.6.JNS11167. Epub 2011 Jul 29
[PubMed PMID: 21800964]
[20]
Williamson RA, Phillips-Bute BG, McDonagh DL, Gray MC, Zomorodi AR, Olson DM, Britz GW, Laskowitz DT, James ML. Predictors of extraventricular drain-associated bacterial ventriculitis. Journal of critical care. 2014 Feb:29(1):77-82. doi: 10.1016/j.jcrc.2013.08.012. Epub 2013 Oct 11
[PubMed PMID: 24125770]
[21]
Schade RP, Schinkel J, Roelandse FW, Geskus RB, Visser LG, van Dijk JM, Voormolen JH, Van Pelt H, Kuijper EJ. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. Journal of neurosurgery. 2006 Jan:104(1):101-8
[PubMed PMID: 16509153]
[22]
Fujikawa A, Tsuchiya K, Honya K, Nitatori T. Comparison of MRI sequences to detect ventriculitis. AJR. American journal of roentgenology. 2006 Oct:187(4):1048-53
[PubMed PMID: 16985156]
[23]
Pfausler B, Spiss H, Beer R, Kampl A, Engelhardt K, Schober M, Schmutzhard E. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. Journal of neurosurgery. 2003 May:98(5):1040-4
[PubMed PMID: 12744364]
Level 1 (high-level) evidence
[24]
Pfausler B, Haring HP, Kampfl A, Wissel J, Schober M, Schmutzhard E. Cerebrospinal fluid (CSF) pharmacokinetics of intraventricular vancomycin in patients with staphylococcal ventriculitis associated with external CSF drainage. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997 Sep:25(3):733-5
[PubMed PMID: 9314470]
[25]
Root BK, Barrena BG, Mackenzie TA, Bauer DF. Antibiotic Impregnated External Ventricular Drains: Meta and Cost Analysis. World neurosurgery. 2016 Feb:86():306-15. doi: 10.1016/j.wneu.2015.09.032. Epub 2015 Sep 25
[PubMed PMID: 26409081]
[26]
Albano S, Berman B, Fischberg G, Siddiqi J, Liu B, Khan Y, Zafar A, Quadri SA, Farooqui M. Retrospective Analysis of Ventriculitis in External Ventricular Drains. Neurology research international. 2018:2018():5179356. doi: 10.1155/2018/5179356. Epub 2018 Sep 2
[PubMed PMID: 30245876]
Level 2 (mid-level) evidence
[27]
Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. The Journal of antimicrobial chemotherapy. 2008 Apr:61(4):869-76. doi: 10.1093/jac/dkn034. Epub 2008 Feb 27
[PubMed PMID: 18305203]
[28]
Atkinson RA, Fikrey L, Vail A, Patel HC. Silver-impregnated external-ventricular-drain-related cerebrospinal fluid infections: a meta-analysis. The Journal of hospital infection. 2016 Mar:92(3):263-72. doi: 10.1016/j.jhin.2015.09.014. Epub 2015 Oct 22
[PubMed PMID: 26601606]
Level 1 (high-level) evidence
[29]
Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, Hamilton AJ. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. Journal of neurosurgery. 2003 Apr:98(4):725-30
[PubMed PMID: 12691395]
Level 1 (high-level) evidence
[30]
Jenkinson MD, Gamble C, Hartley JC, Hickey H, Hughes D, Blundell M, Griffiths MJ, Solomon T, Mallucci CL. The British antibiotic and silver-impregnated catheters for ventriculoperitoneal shunts multi-centre randomised controlled trial (the BASICS trial): study protocol. Trials. 2014 Jan 3:15():4. doi: 10.1186/1745-6215-15-4. Epub 2014 Jan 3
[PubMed PMID: 24383496]
Level 1 (high-level) evidence
[31]
Winfield JA, Rosenthal P, Kanter RK, Casella G. Duration of intracranial pressure monitoring does not predict daily risk of infectious complications. Neurosurgery. 1993 Sep:33(3):424-30; discussion 430-1
[PubMed PMID: 8413873]
Level 3 (low-level) evidence
[32]
Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, Jane JA, Ward JD, Young HF, Marmarou A. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. Journal of neurosurgery. 1996 Sep:85(3):419-24
[PubMed PMID: 8751626]
[33]
Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. Journal of neurology, neurosurgery, and psychiatry. 2002 Dec:73(6):759-61
[PubMed PMID: 12438486]
Level 1 (high-level) evidence
[34]
Shang F, Xu Y, Wang N, Cheng W, Chen W, Duan W. Diagnosis and treatment of severe neurosurgical patients with pyogenic ventriculitis caused by gram-negative bacteria. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2018 Jan:39(1):79-84. doi: 10.1007/s10072-017-3146-8. Epub 2017 Oct 13
[PubMed PMID: 29027589]
[35]
Mactier H, Galea P, McWilliam R. Acute obstructive hydrocephalus complicating bacterial meningitis in childhood. BMJ (Clinical research ed.). 1998 Jun 20:316(7148):1887-9
[PubMed PMID: 9632412]
[36]
Alleyne CH Jr, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000 Nov:47(5):1124-7; discussion 1127-9
[PubMed PMID: 11063105]
[37]
Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta neurochirurgica. Supplement. 1998:71():146-8
[PubMed PMID: 9779169]
Level 1 (high-level) evidence
[38]
Bader MK, Littlejohns L, Palmer S. Ventriculostomy and intracranial pressure monitoring: in search of a 0% infection rate. Heart & lung : the journal of critical care. 1995 Mar-Apr:24(2):166-72
[PubMed PMID: 7759277]
[39]
Brown EM. Antimicrobial prophylaxis in neurosurgery. The Journal of antimicrobial chemotherapy. 1993 Feb:31 Suppl B():49-63
[PubMed PMID: 8449846]
[40]
Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2008 Feb:62 Suppl 2():532-9. doi: 10.1227/01.neu.0000316256.44349.b1. Epub
[PubMed PMID: 18596451]
[41]
Lord AS, Nicholson J, Lewis A. Infection Prevention in the Neurointensive Care Unit: A Systematic Review. Neurocritical care. 2019 Aug:31(1):196-210. doi: 10.1007/s12028-018-0568-y. Epub
[PubMed PMID: 29998427]
Level 1 (high-level) evidence