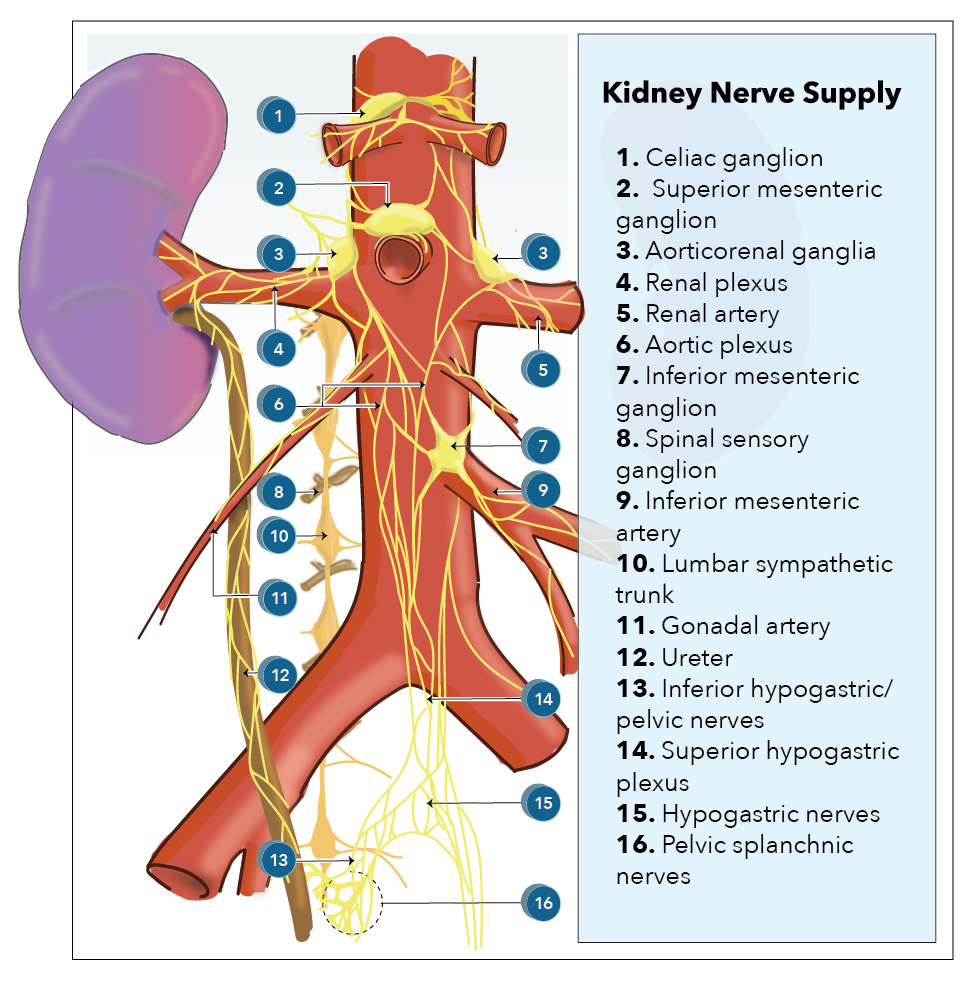
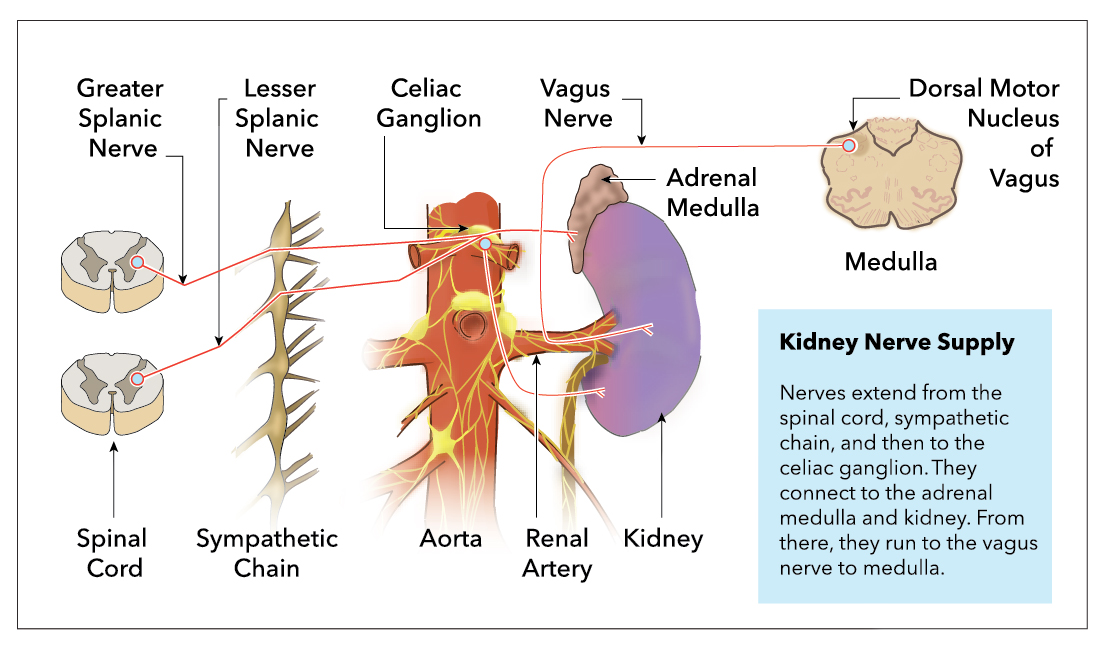
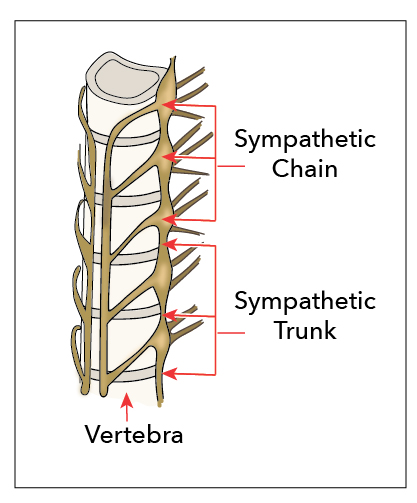
[1]
DiBona GF, Kopp UC. Neural control of renal function. Physiological reviews. 1997 Jan:77(1):75-197
[PubMed PMID: 9016301]
[2]
Becker BK, Zhang D, Soliman R, Pollock DM. Autonomic nerves and circadian control of renal function. Autonomic neuroscience : basic & clinical. 2019 Mar:217():58-65. doi: 10.1016/j.autneu.2019.01.003. Epub 2019 Jan 10
[PubMed PMID: 30704976]
[3]
Maeda S, Kuwahara-Otani S, Tanaka K, Hayakawa T, Seki M. Origin of efferent fibers of the renal plexus in the rat autonomic nervous system. The Journal of veterinary medical science. 2014 May:76(5):763-5
[PubMed PMID: 24430660]
[4]
Ciriello J. Afferent renal inputs onto subfornical organ neurons responsive to angiotensin II. The American journal of physiology. 1997 May:272(5 Pt 2):R1684-9
[PubMed PMID: 9176365]
[5]
Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Current vascular pharmacology. 2014:12(6):845-58
[PubMed PMID: 24066938]
[6]
Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Journal of managed care pharmacy : JMCP. 2007 Oct:13(8 Suppl B):9-20
[PubMed PMID: 17970613]
[7]
Seely JC. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: juvenile animal relevancy. Journal of toxicologic pathology. 2017 Apr:30(2):125-133. doi: 10.1293/tox.2017-0006. Epub 2017 Feb 11
[PubMed PMID: 28458450]
Level 3 (low-level) evidence
[8]
Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Seminars in nephrology. 2009 Jul:29(4):321-37. doi: 10.1016/j.semnephrol.2009.03.009. Epub
[PubMed PMID: 19615554]
[11]
Singh RR, McArdle ZM, Iudica M, Easton LK, Booth LC, May CN, Parkington HC, Lombardo P, Head GA, Lambert G, Moritz KM, Schlaich MP, Denton KM. Sustained Decrease in Blood Pressure and Reduced Anatomical and Functional Reinnervation of Renal Nerves in Hypertensive Sheep 30 Months After Catheter-Based Renal Denervation. Hypertension (Dallas, Tex. : 1979). 2019 Mar:73(3):718-727. doi: 10.1161/HYPERTENSIONAHA.118.12250. Epub
[PubMed PMID: 30661475]
[12]
Nishi EE, Lopes NR, Gomes GN, Perry JC, Sato AYS, Naffah-Mazzacoratti MG, Bergamaschi CT, Campos RR. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertension research : official journal of the Japanese Society of Hypertension. 2019 May:42(5):628-640. doi: 10.1038/s41440-018-0171-9. Epub 2018 Dec 20
[PubMed PMID: 30573809]
[14]
Siccardi MA, Tariq MA, Valle C. Anatomy, Bony Pelvis and Lower Limb: Psoas Major. StatPearls. 2023 Jan:():
[PubMed PMID: 30571039]
[16]
Loukas M, Klaassen Z, Merbs W, Tubbs RS, Gielecki J, Zurada A. A review of the thoracic splanchnic nerves and celiac ganglia. Clinical anatomy (New York, N.Y.). 2010 Jul:23(5):512-22. doi: 10.1002/ca.20964. Epub
[PubMed PMID: 20235178]
[18]
Costa A, Matter M, Pascual M, Doerfler A, Venetz JP. [Renal, vascular and urological variations and abnormalities in living kidney donor candidates]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2019 Mar:29(3):166-172. doi: 10.1016/j.purol.2018.12.001. Epub 2019 Jan 28
[PubMed PMID: 30704916]
[19]
Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiological reviews. 2015 Apr:95(2):405-511. doi: 10.1152/physrev.00042.2012. Epub
[PubMed PMID: 25834230]
[20]
Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Frontiers in medicine. 2018:5():82. doi: 10.3389/fmed.2018.00082. Epub 2018 Mar 29
[PubMed PMID: 29651418]