Continuing Education Activity
Shoulder pain is a common patient complaint encountered both by primary care physicians and orthopedic surgeons. Many shoulder structures can account for the clinical presentation of shoulder pain, and the diagnosis can be challenging. Clinicians often use a combination of physical examination, clinical history, and imaging to establish a diagnosis; however, sometimes, the diagnosis remains in question. An ultrasound-guided injection can aid in these cases providing clinical information and often therapeutic benefit. This activity reviews the indications, potential complications, and the method for performing an ultrasound-guided biceps tendon sheath injection.
Objectives:
- Review shoulder anatomy with a focus on the biceps tendon.
- Outline the clinical presentation of patients with biceps tendinosis or superior labral tear.
- Identify the indications for ultrasound-guided biceps injection.
- Summarize the procedure for ultrasound-guided biceps tendon injection.
Introduction
Ultrasound (US) is well suited for use in clinical practice given its affordability, portability, and lack of radiation exposure for image acquisition. These features allow clinicians from almost any specialty the ability to perform image-guided evaluations and interventions.[1][2][3][4][5] Radiologists are uniquely suited to perform these types of evaluations and procedures given their extensive training, knowledge of human anatomy, and understanding of varied anatomical appearances on multiple imaging modalities.[6]
Part of a radiologist’s training comprises an understanding of medical physics, including how medical images are obtained. In ultrasound, the sonographer or physician utilizes a transducer to interrogate the area of interest. Within the transducer are piezoelectric crystals that vibrate when exposed to an alternating current. These vibrations generate sound waves transmitted into the patient’s soft tissues via a layer of ultrasound gel. The sound waves then interact with the tissue and are ultimately reflected back to the transducer. Once the sound waves return to the transducer, they are converted back into an electric current. The computer then calculates the time interval between when the sound wave was initially generated and when it was received back at the transducer to determine the location (depth) of the tissue, which reflected the sound wave.
When planning a procedure, one must consider the type of transducer which will yield the highest quality images. There are a variety of transducers that can be used with varied frequency and shape. In general, higher frequency transducers are utilized for imaging more superficial structures as they offer better resolution. However, the higher frequency sound wave is more easily attenuated and has decreased ability to penetrate tissue, limiting their use in the evaluation of deeper structures. For deeper structures or larger patients, one might choose a lower frequency or curved transducer. When imaging small body parts, such as a finger, a small transducer (hockey stick) may help.
Image guidance for procedures includes the use of ultrasound, fluoroscopy, computed tomography (CT), or in some cases, MRI. In general, ultrasound is particularly helpful in the guidance of soft tissue procedures such as therapeutic tendon sheath injection, soft tissue biopsy, as well as cyst or abscess aspiration/drainage.[1][7] Ultrasound, CT, and fluoroscopy guidance can be used for both joint aspiration and therapeutic injection. The chosen modality may depend on availability, user expertise, the body part to be treated, and patient body habitus. Advantages to US include lack of ionizing radiation and direct real-time visualization of the needle and surrounding soft tissues during the procedure. During fluoroscopic guidance, the needle is intermittently imaged, and its position is judged only in relation to the associated osseous structures. The involved soft tissue structures must be inferred by knowledge of anatomy. Also, fluoroscopy often requires a contrast agent to verify needle placement as opposed to US, which allows for direct visualization of the needle position. CT is useful for biopsy of osseous structures or therapeutic injection of deep anatomic structures, which can be difficult to visualize with US or palpate on physical examination.[7][8][9][10]
Anatomy and Physiology
The long head biceps muscle has a proximal insertion at the shoulder where the tendon is intra-articular, originating on the supraglenoid tubercle and superior glenoid labrum. It exits the glenohumeral joint at the level of the humeral head near the rotator cuff attachments on the humerus, entering the bicipital groove. It is held within the proximal bicipital groove by the coracohumeral and transverse humeral ligaments as well as fibers of the subscapularis tendon.
In most patients, there are distinct attachments of the long and short head of the biceps tendon on the radial tuberosity. The distal biceps tendons traverse the elbow joint and attach to the radial tuberosity. The long head normally constitutes the more proximal insertion on the radial tuberosity and the short head more distal. Therefore it is possible to have an isolated tear of either the long or short head.
At the distal biceps myotendinous junction, there is a broad aponeurosis known as the lacertus fibrosus (also called the bicipital aponeurosis), which circumferentially covers the flexor muscle attachments as well as the median nerve and brachial artery. This aponeurosis originates at the distal biceps tendon and inserts onto the radial tuberosity and proximal ulna. It is held in place by multiple strong fascial connections and serves as a protective covering for the neurovascular structures which course beneath. In most cases, the lacertus fibrosus prevents a torn biceps tendon from retracting more than a few centimeters proximal to the elbow joint; however, if the lacertus fibrosus is also injured, the tendon retraction may be more severe, leading to a more difficult surgical repair.
Indications
Ultrasound has uses in a wide range of musculoskeletal procedures, including joint or bursa aspiration to evaluate for infection or crystalline deposition disease, joint or bursa injection such as an arthrogram or steroid injection, tendon sheath injection, tendinosis therapy such as dry needling, platelet-rich plasma (PRP) injection, and percutaneous biopsy. Injections into a joint, bursa, or tendon sheath can be both therapeutic (pain relief) and diagnostic by allowing the referring provider to gain knowledge of the patient’s pathology based on their pain response post-treatment.[11][12]
Shoulder pain can be complex and multifactorial. Clinicians use a combination of physical examination, history, and imaging to establish a differential diagnosis.[13][14] In some cases, MRI imaging may identify multiple abnormal findings and injections to help elucidate the clinical significance of the imaging findings. For example, the MRI examination may identify a superior labral tear and biceps tendinosis in addition to rotator cuff tear, but the physical examination is most consistent with rotator cuff pathology. The orthopedist may request a biceps tendon injection to determine if a biceps tenodesis at the time of rotator cuff repair is indicated.[6][15][16]
Contraindications
Absolute contraindications to corticosteroid injections include local infection and bacteremia. If the injection is intraarticular, absolute contraindications would also include septic arthrosis and intraarticular fracture. In the setting of biceps tendon sheath injection, it should be noted that the biceps tendon sheath communicates with the glenohumeral joint. Therefore, medication injected into the biceps tendon sheath will also extend to the joint. If the clinical indication is to assess for septic arthrosis or tenosynovitis, one must carefully evaluate the overlying soft tissues for signs of cellulitis. It is crucial to avoid any potential contamination of the joint or tendon sheath, which could occur by placing the needle through infected soft tissue before accessing the joint. Relative contraindications include local or juxta-articular osteoporosis, coagulopathy, or recent injection or multiple frequent injections, defined as three times over the last year or within the prior six weeks.[17]
Equipment
Several varieties of steroids are available for use on the market today, including methylprednisolone acetate, triamcinolone, betamethasone acetate with betamethasone sodium phosphate, and dexamethasone sodium phosphate, each of which offers their strengths and weakness. The most commonly used steroids in the United States include methylprednisolone, triamcinolone hexacetonide, triamcinolone acetonide, and betamethasone.[18]
Of note, the equivalent potency of betamethasone and dexamethasone sodium phosphate is approximately five times that of the others. Solubility also varies between the steroid formulations. For example, the acetate portion of betamethasone is insoluble, while the sodium phosphate portion of betamethasone and dexamethasone sodium phosphate is freely water-soluble. Particle size varies from 0.5 micrometers dexamethasone to over 500 micrometers methylprednisolone acetate, and triamcinolone has extensive particle aggregation, which requires meticulous mixing of the solution before injection.
Dosage varies based on the chosen steroid and location treated with small joints (metacarpophalangeal, facet, acromioclavicular), medium-sized joints (elbow, wrist), and larger joints (knee, shoulder, hip, ankle) receiving respectively increased amounts of steroid. For example, while a large joint might require 40 to 80mg of methylprednisolone acetate, that same joint would only require 8 to 15 mg of dexamethasone sodium phosphate. Similarly, a small joint might require 4 to 10 mg of methylprednisolone acetate, while that same joint would only require 0.8 to 2 mg of dexamethasone sodium phosphate. Some examples of steroid mixtures used for biceps tendon sheath injection at our institution include 3.75 mg dexamethasone mixed with 2 ml 1% lidocaine or 1ml betamethasone (6 mg/ml) mixed with 2 ml 1% lidocaine.[19]
Before injecting the steroid, a local anesthetic is provided to the skin and superficial soft tissues using 1% lidocaine. Although around 5 to 7 mL is the typical amount for local anesthesia, it bears mentioning that the maximum safe dosage for lidocaine in the United States is 300 mg, which equates to 30 mL of 1% lidocaine.[18] Bupivacaine is an alternate medication that can be useful for anesthesia. Its long duration of action is advantageous, but it has shown the highest toxicity to chondrocytes of the local anesthetics. It should never be used for intraarticular injections.[11][12][18][20][21]
Preparation
As with every image-guided procedure, planning begins with reviewing the patient’s history, clinical indication, and relevant prior imaging, including plain film, ultrasound, CT, and MRI. After reviewing the history and available imaging, a decision should be made about whether the procedure requested is medically indicated and if it is the best procedure available to address the clinical concern. All medications should be reviewed with specific attention to the use of any anticoagulation therapy, blood thinners (including fish oil and vitamin E), and aspirin use. Risks and benefits for holding or continuing anticoagulation should be considered on a case-by-case basis and may involve discussion with the prescribing provider.[5][22] Additionally, one needs to review the patient’s allergies to ensure there are no prior reactions to any of the medications planned for use, including skin cleansing agents and adhesives.
Once the requested procedure is determined to be clinically indicated, the patient should be made aware of the risks, benefits, and alternatives. While nearly all musculoskeletal injections are considered low risk, some potential risks of ultrasound-guided procedures include pain, infection, bleeding, damage to nearby structures, allergic reaction, steroid flare, and failure to address the clinical concern (e.g., failure to relieve pain). Additionally, if steroids are inadvertently injected into the subcutaneous soft tissues, atrophy of the subcutaneous fat or skin discoloration can occur. Although extremely rare, it has been documented in the literature that steroid injections can weaken tendons; thus, there is a theoretical risk of tendon rupture.[18] Once this discussion is completed and all the patient’s questions are answered to their satisfaction, written consent must be obtained. If the procedure is performed for pain relief, a pre and post-procedural pain level should also be recorded to document an objective measurement of symptomatic relief. Following the procedure and before releasing the patient from the radiology department, the patient is given return precautions such as those which might indicate a post-procedural infection, including worsening pain beyond the baseline, redness, swelling, and/or fever. Although exceedingly rare, an intraarticular joint infection would require urgent medical attention. Thus patients are instructed to seek care in urgent care or emergency department if symptoms present outside of normal business hours.
Technique or Treatment
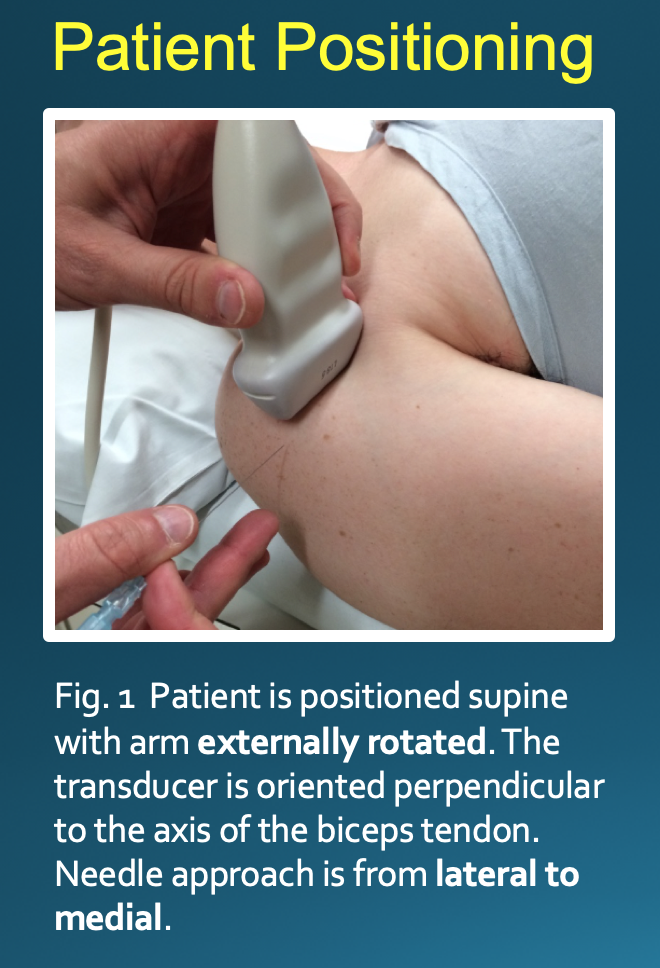
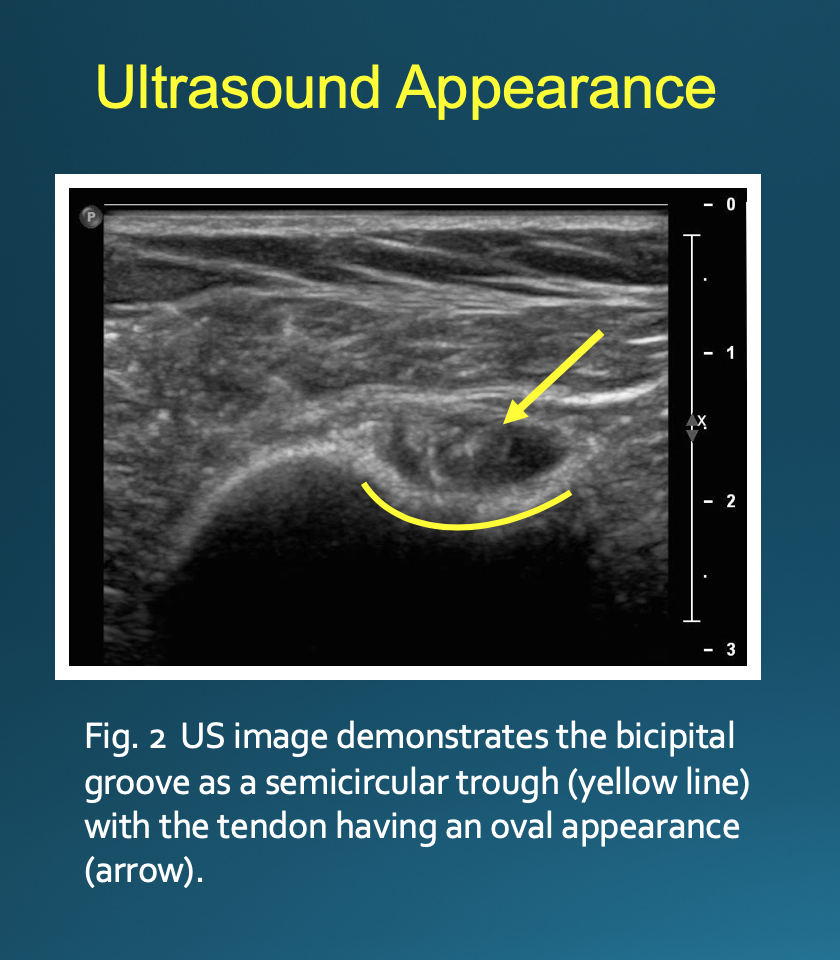
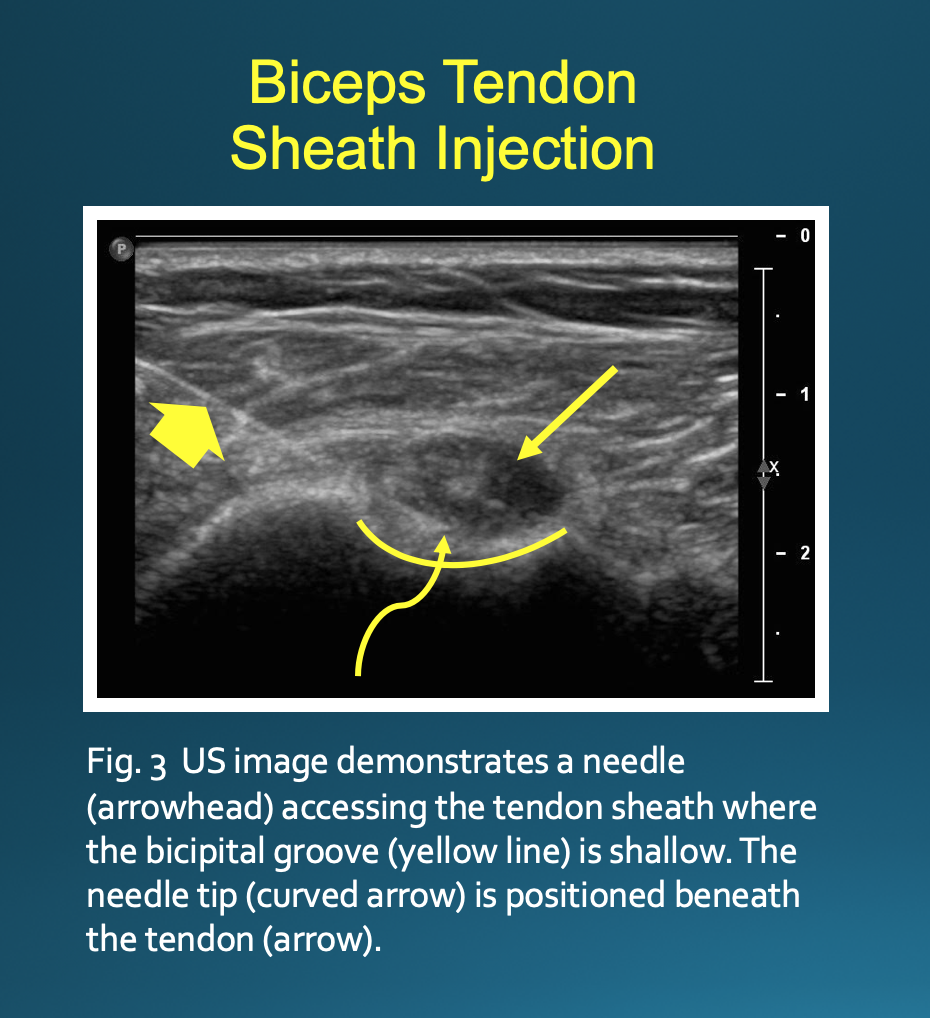
Optimal positioning can be patient-dependent; however, most patients will be positioned supine with their arm externally rotated. The transducer is placed on the skin perpendicular to the axis of the biceps tendon (Fig. 1). This will result in an image with the bicipital groove appearing as a semicircular trough with the tendon having a circular or oval appearance (Fig. 2). The approach is from lateral to medial with an in-plane technique. The patient’s skin is marked at the planned injection site. The skin is then prepped and draped in a sterile fashion. A sterile US probe cover is placed on the selected probe. The subcutaneous soft tissues are then infiltrated with 1% lidocaine as the needle is advanced towards the biceps tendon under US guidance. Optimally, one should enter the tendon sheath in an area where the bicipital groove is shallow, allowing the needle to be positioned somewhat parallel to the transducer. The more parallel the needle is to the transducer, the more clearly it will be visualized on US. A steeper approach will lead to decreased reflection of sound waves returning to the transducer and poorer visualization. If the needle tip is positioned superficial to the tendon, the subdeltoid bursa can fill with fluid mimicking the tendon sheath; thus, the ideal positioning is beneath the tendon to avoid this pitfall (Fig. 3).
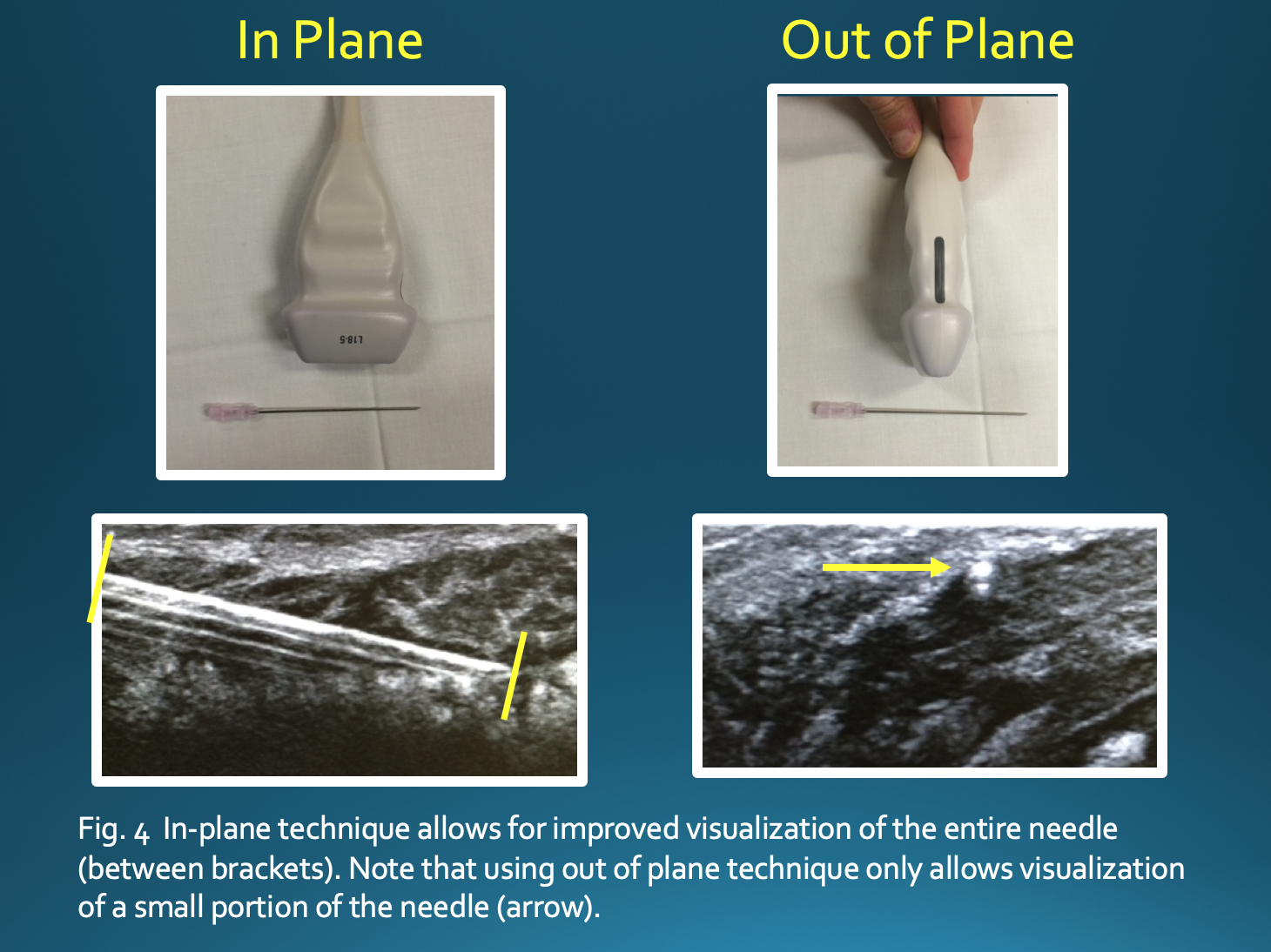
The in-plane technique, in which there is a parallel orientation of the needle with respect to the transducer, allows for improved visualization of the entire needle and limits the risk of damage to surrounding structures. If using an out-of-plane technique, one would only see a small portion of the needle, making it difficult to appreciate the position of the needle tip (Fig 4). Some options to increase needle visibility include beam steering, heel-toe maneuver, use of a larger gauge needle, and entering the skin farther from the target, which allows for more parallel orientation but a longer needle path.[23][24]
Given the superficial nature of the biceps tendon, the use of a higher frequency ultrasound transducer (10 to 15 MHz) will allow for better near field resolution. The probe should be maintained perpendicularly to the tendon to maximize the reflection of sound beams and decrease anisotropy, which can result in poor visualization of the tendon.
Complications
Potential complications include pain, infection, bleeding, damage to nearby structures, allergic reaction, and non-therapeutic results. Complications specific to steroid use include septic arthritis, post-injection flare, local tissue atrophy, skin discoloration, tendon rupture, cartilage damage, avascular necrosis, flushing, and increased blood glucose level. If inadvertently injected intravascularly, there can be severe CNS and cardiac effects, particularly with insoluble, particulate formulations such as triamcinolone, which can embolize and lead to stroke.[18] As mentioned previously, the maximum safe dose of lidocaine in the US is 300 mg or 30 ml of 1% lidocaine. Above this threshold, there is a risk of CNS, cardiac, and skeletal muscle toxicity. Local anesthetics have been shown to be toxic to chondrocytes in both animal studies and in vitro studies using human chondrocytes; bupivacaine is the most chondrotoxic, and ropivacaine the least. Lidocaine is most often used because it is the least expensive and most readily available.[20][21]
Enhancing Healthcare Team Outcomes
As mentioned above, shoulder pain is often multifactorial in etiology. Often the radiologist and orthopedist work together to determine the underlying cause(s) of a patient’s pain. A patient will typically present initially to their primary care provider, and if clinically indicated, they may be referred to an orthopedic specialist for further evaluation. Considering the patient’s history and physical exam findings, further evaluation with diagnostic imaging is sometimes indicated to evaluate for underlying pathology. It is not uncommon for imaging evaluation, such as MRI, to demonstrate multiple abnormalities, any of which could be causing the patient’s pain. In these cases, it can help perform a diagnostic and sometimes therapeutic injection to help clarify the clinical significance of the imaging findings. If a patient has pain relief from a targeted injection, it can be inferred that the area treated was a pain generator. Alternatively, if the patient does not have pain relief, one can infer that this area was not a pain generator. The patient’s response is clinically important as it can guide what surgical procedures, if any, may be offered for symptomatic relief. It can also help a patient avoid unnecessary surgical procedures. Orthopedic nurses set up and assist during the procedure, provide patient instruction. Physical therapy may need to assist with post-procedure recovery exercises and strengthening the muscles around the shoulder joint. These various disciplines need to function as an interprofessional team to bring about optimal patient results from this procedure. [Level 5]