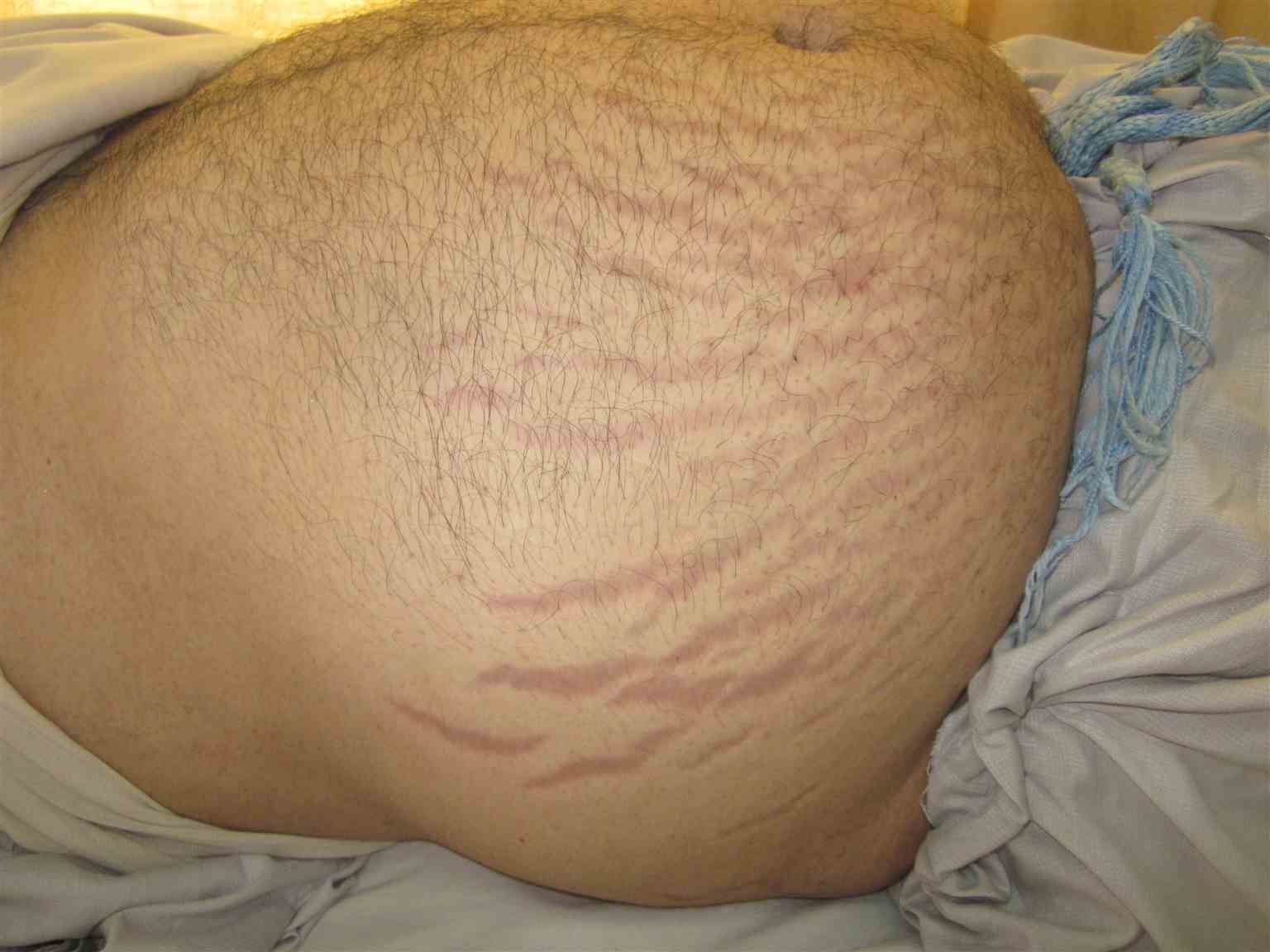
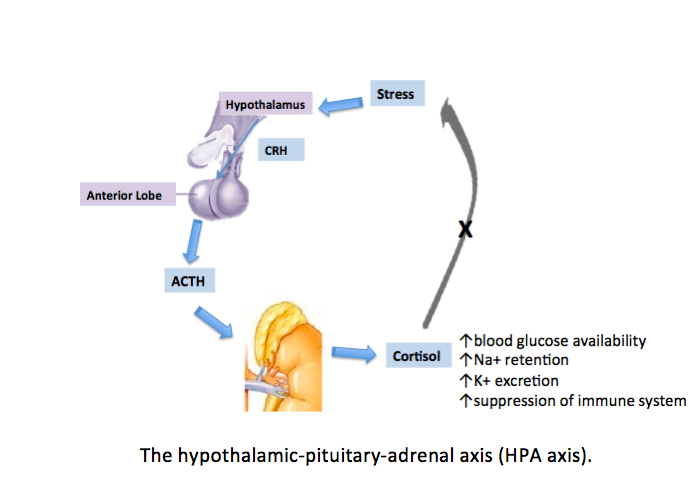
[1]
Hine J,Schwell A,Kairys N, An Unlikely Cause of Hypokalemia. The Journal of emergency medicine. 2017 May
[PubMed PMID: 28139270]
[2]
Buliman A,Tataranu LG,Paun DL,Mirica A,Dumitrache C, Cushing's disease: a multidisciplinary overview of the clinical features, diagnosis, and treatment. Journal of medicine and life. 2016 Jan-Mar
[PubMed PMID: 27974908]
Level 3 (low-level) evidence
[3]
Hanna FWF,Issa BG,Kevil B,Fryer AA, Investigating cortisol excess or deficiency: a practical approach. BMJ (Clinical research ed.). 2019 Nov 26
[PubMed PMID: 31771937]
[4]
Annapureddy AR,Angraal S,Caraballo C,Grimshaw A,Huang C,Mortazavi BJ,Krumholz HM, The National Institutes of Health funding for clinical research applying machine learning techniques in 2017. NPJ digital medicine. 2020
[PubMed PMID: 32025574]
[6]
Schorr M,Zhang X,Zhao W,Abedi P,Lines KE,Hedley-Whyte ET,Swearingen B,Klibanski A,Miller KK,Thakker RV,Nachtigall LB, TWO SYNCHRONOUS PITUITARY ADENOMAS CAUSING CUSHING DISEASE AND ACROMEGALY. AACE clinical case reports. 2019 Sep-Oct
[PubMed PMID: 31967052]
Level 3 (low-level) evidence
[7]
Trabzonlu L,Agirlar Trabzonlu T,Gurbuz Y,Ceylan S, ACTH-Cell Pituitary Adenoma With Signet Ring Cells: A Rare Case Report and Review of The Literature. Applied immunohistochemistry & molecular morphology : AIMM. 2020 Feb
[PubMed PMID: 32044887]
Level 3 (low-level) evidence
[8]
Pappachan JM,Hariman C,Edavalath M,Waldron J,Hanna FW, Cushing's syndrome: a practical approach to diagnosis and differential diagnoses. Journal of clinical pathology. 2017 Apr
[PubMed PMID: 28069628]
[9]
Webb SM,Santos A,Aulinas A,Resmini E,Martel L,Martínez-Momblán MA,Valassi E, Patient-Centered Outcomes with Pituitary and Parasellar Disease. Neuroendocrinology. 2020
[PubMed PMID: 32101858]
[10]
Magnetic resonance imaging and histology correlation in Cushing's disease., Masopust V,Netuka D,Beneš V,Májovský M,Belšán T,Bradáč O,Hořínek D,Kosák M,Hána V,Kršek M,, Neurologia i neurochirurgia polska, 2017 Jan - Feb
[PubMed PMID: 27988033]
[11]
Recent Progress in the Medical Therapy of Pituitary Tumors., Langlois F,McCartney S,Fleseriu M,, Endocrinology and metabolism (Seoul, Korea), 2017 Jun
[PubMed PMID: 28685507]
[12]
Cushing Syndrome: Diagnostic Workup and Imaging Features, With Clinical and Pathologic Correlation., Wagner-Bartak NA,Baiomy A,Habra MA,Mukhi SV,Morani AC,Korivi BR,Waguespack SG,Elsayes KM,, AJR. American journal of roentgenology, 2017 Jul
[PubMed PMID: 28639924]
[13]
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, Casanueva FF, Castinetti F, Chanson P, Findling J, Gadelha M, Geer EB, Giustina A, Grossman A, Gurnell M, Ho K, Ioachimescu AG, Kaiser UB, Karavitaki N, Katznelson L, Kelly DF, Lacroix A, McCormack A, Melmed S, Molitch M, Mortini P, Newell-Price J, Nieman L, Pereira AM, Petersenn S, Pivonello R, Raff H, Reincke M, Salvatori R, Scaroni C, Shimon I, Stratakis CA, Swearingen B, Tabarin A, Takahashi Y, Theodoropoulou M, Tsagarakis S, Valassi E, Varlamov EV, Vila G, Wass J, Webb SM, Zatelli MC, Biller BMK. Consensus on diagnosis and management of Cushing's disease: a guideline update. The lancet. Diabetes & endocrinology. 2021 Dec:9(12):847-875. doi: 10.1016/S2213-8587(21)00235-7. Epub 2021 Oct 20
[PubMed PMID: 34687601]
Level 3 (low-level) evidence
[14]
Lu L,Chen JH,Zhu HJ,Song AL,Li M,Chen S,Pan H,Gong FY,Wang RZ,Xing B,Yao Y,Feng M,Lu ZL, [Comparison of efficacy between the serum cortisol and 24 hour urine free cortisol in combined dexamethasone suppression test in the diagnosis of Cushing syndrome]. Zhonghua yi xue za zhi. 2016 Jul 19
[PubMed PMID: 27464539]
[16]
Braun LT,Riester A,Oßwald-Kopp A,Fazel J,Rubinstein G,Bidlingmaier M,Beuschlein F,Reincke M, Toward a Diagnostic Score in Cushing's Syndrome. Frontiers in endocrinology. 2019
[PubMed PMID: 31787931]
[17]
Vassiliadi DA,Balomenaki M,Asimakopoulou A,Botoula E,Tzanela M,Tsagarakis S, The Desmopressin Test Predicts Better Than Basal Cortisol the Long-Term Surgical Outcome of Cushing's Disease. The Journal of clinical endocrinology and metabolism. 2016 Dec
[PubMed PMID: 27662440]
[18]
Lad SP,Patil CG,Laws ER Jr,Katznelson L, The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing's disease. Neurosurgical focus. 2007
[PubMed PMID: 17961020]
[19]
Losa M,Albano L,Bailo M,Barzaghi LR,Mortini P, Role of radiosurgery in the treatment of Cushing's disease. Journal of neuroendocrinology. 2022 Aug
[PubMed PMID: 35980263]
[20]
Ye VC,Akagami R, Perioperative Quality of Life in Cushing's Disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2017 Jan
[PubMed PMID: 27645104]
Level 2 (mid-level) evidence
[22]
Ciato D,Mumbach AG,Paez-Pereda M,Stalla GK, Currently used and investigational drugs for Cushing´s disease. Expert opinion on investigational drugs. 2017 Jan
[PubMed PMID: 27894193]
Level 3 (low-level) evidence
[23]
Target delineation and optimal radiosurgical dose for pituitary tumors., Minniti G,Osti MF,Niyazi M,, Radiation oncology (London, England), 2016 Oct 11
[PubMed PMID: 27729088]
[24]
Long-term outcomes of children treated for Cushing's disease: a single center experience., Yordanova G,Martin L,Afshar F,Sabin I,Alusi G,Plowman NP,Riddoch F,Evanson J,Matson M,Grossman AB,Akker SA,Monson JP,Drake WM,Savage MO,Storr HL,, Pituitary, 2016 Dec
[PubMed PMID: 27678103]
[25]
Honegger J,Nasi-Kordhishti I, Surgery and perioperative management of patients with Cushing's disease. Journal of neuroendocrinology. 2022 Aug
[PubMed PMID: 35980172]
[26]
Abellán Galiana P,Fajardo Montañana C,Riesgo Suárez PA,Gómez Vela J,Escrivá CM,Lillo VR, [Predictors of long-term remission after transsphenoidal surgery in Cushing's disease]. Endocrinologia y nutricion : organo de la Sociedad Espanola de Endocrinologia y Nutricion. 2013 Oct;
[PubMed PMID: 23266144]
[27]
Kuo CH,Shih SR,Li HY,Chen SC,Hung PJ,Tseng FY,Chang TC, Adrenocorticotropic hormone levels before treatment predict recurrence of Cushing's disease. Journal of the Formosan Medical Association = Taiwan yi zhi. 2017 Jun
[PubMed PMID: 28029519]
[28]
Bertherat J, THE CHALLENGE OF EARLY DIAGNOSIS OF CUSHING DISEASE RECURRENCE! Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Nov
[PubMed PMID: 27749130]
[29]
Fleseriu M,Hamrahian AH,Hoffman AR,Kelly DF,Katznelson L,AACE Neuroendocrine and Pituitary Scientific Committee *., AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY DISEASE STATE CLINICAL REVIEW: DIAGNOSIS OF RECURRENCE IN CUSHING DISEASE. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Dec
[PubMed PMID: 27643842]
[30]
Grabner P,Hauer-Jensen M,Jervell J,Flatmark A, Long-term results of treatment of Cushing's disease by adrenalectomy. The European journal of surgery = Acta chirurgica. 1991 Aug
[PubMed PMID: 1681932]
[31]
Ferriere A,Cortet C,Chanson P,Delemer B,Caron P,Chabre O,Reznik Y,Bertherat J,Rohmer V,Briet C,Raingeard I,Castinetti F,Beckers A,Vroonen L,Maiter D,Cephise-Velayoudom FL,Nunes ML,Haissaguerre M,Tabarin A, Cabergoline for Cushing's disease: a large retrospective multicenter study. European journal of endocrinology. 2017 Mar
[PubMed PMID: 28007845]
Level 2 (mid-level) evidence
[32]
von Selzam V,Theodoropoulou M, Innovative tumour targeting therapeutics in Cushing's disease. Best practice & research. Clinical endocrinology & metabolism. 2022 Dec
[PubMed PMID: 36511278]
[33]
Lacroix A,Gu F,Gallardo W,Pivonello R,Yu Y,Witek P,Boscaro M,Salvatori R,Yamada M,Tauchmanova L,Roughton M,Ravichandran S,Petersenn S,Biller BMK,Newell-Price J,Pasireotide G2304 Study Group, Efficacy and safety of once-monthly pasireotide in Cushing's disease: a 12 month clinical trial. The lancet. Diabetes & endocrinology. 2018 Jan
[PubMed PMID: 29032078]
[34]
Lacroix A,Bronstein MD,Schopohl J,Delibasi T,Salvatori R,Li Y,Barkan A,Suzaki N,Tauchmanova L,Ortmann CE,Ravichandran S,Petersenn S,Pivonello R, Long-acting pasireotide improves clinical signs and quality of life in Cushing
[PubMed PMID: 32385851]
Level 2 (mid-level) evidence
[35]
Hinojosa-Amaya JM,Johnson N,González-Torres C,Varlamov EV,Yedinak CG,McCartney S,Fleseriu M, Depression and Impulsivity Self-Assessment Tools to Identify Dopamine Agonist Side Effects in Patients With Pituitary Adenomas. Frontiers in endocrinology. 2020
[PubMed PMID: 33193096]
[36]
Castinetti F,Guignat L,Giraud P,Muller M,Kamenicky P,Drui D,Caron P,Luca F,Donadille B,Vantyghem MC,Bihan H,Delemer B,Raverot G,Motte E,Philippon M,Morange I,Conte-Devolx B,Quinquis L,Martinie M,Vezzosi D,Le Bras M,Baudry C,Christin-Maitre S,Goichot B,Chanson P,Young J,Chabre O,Tabarin A,Bertherat J,Brue T, Ketoconazole in Cushing's disease: is it worth a try? The Journal of clinical endocrinology and metabolism. 2014 May
[PubMed PMID: 24471573]
[37]
Young J,Bertherat J,Vantyghem MC,Chabre O,Senoussi S,Chadarevian R,Castinetti F,Compassionalte use Programme, Hepatic safety of ketoconazole in Cushing
[PubMed PMID: 29472378]
[38]
Rasool S,Skinner BW, Osilodrostat for the treatment of Cushing's disease. Expert opinion on pharmacotherapy. 2021 Jun
[PubMed PMID: 33703978]
Level 3 (low-level) evidence
[39]
Pivonello R,Fleseriu M,Newell-Price J,Bertagna X,Findling J,Shimatsu A,Gu F,Auchus R,Leelawattana R,Lee EJ,Kim JH,Lacroix A,Laplanche A,O'Connell P,Tauchmanova L,Pedroncelli AM,Biller BMK,LINC 3 investigators, Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. The lancet. Diabetes & endocrinology. 2020 Sep
[PubMed PMID: 32730798]
Level 1 (high-level) evidence
[40]
Fleseriu M,Findling JW,Koch CA,Schlaffer SM,Buchfelder M,Gross C, Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. The Journal of clinical endocrinology and metabolism. 2014 Oct
[PubMed PMID: 25013998]
[41]
Guarda FJ,Findling J,Yuen KCJ,Fleseriu M,Nachtigall LB, Mifepristone Increases Thyroid Hormone Requirements in Patients With Central Hypothyroidism: A Multicenter Study. Journal of the Endocrine Society. 2019 Sep 1;
[PubMed PMID: 31528830]
Level 2 (mid-level) evidence
[42]
Coulden A, Hamblin R, Wass J, Karavitaki N. Cardiovascular health and mortality in Cushing's disease. Pituitary. 2022 Oct:25(5):750-753. doi: 10.1007/s11102-022-01258-4. Epub 2022 Jul 22
[PubMed PMID: 35869339]
[43]
[Serum lipids in diminished kidney function, during hemodialysis and after kidney transplantation]., Frey FJ,Mordasini RC,Schlumpf E,Marone C,Montandon A,Flury W,Riva G,, Schweizerische medizinische Wochenschrift, 1977 Sep 10
[PubMed PMID: 31471057]
[44]
Vega-Beyhart A,Enriquez-Estrada VM,Bello-Chavolla OY,Torres-Victoria TR,Martínez-Sánchez FD,López-Navarro JM,Pérez-Guzmán MC,Hinojosa-Amaya JM,León-Suárez A,Espinoza-Salazar HD,Roldán-Sarmiento P,Gómez-Sámano MA,Gómez-Pérez FJ,Cuevas-Ramos D, Quality of life is significantly impaired in both secretory and non-functioning pituitary adenomas. Clinical endocrinology. 2019 Mar;
[PubMed PMID: 30548674]
Level 2 (mid-level) evidence
[45]
Rotman LE,Vaughan TB,Hackney JR,Riley KO, Long-Term Survival After Transformation of an Adrenocorticotropic Hormone-Secreting Pituitary Macroadenoma to a Silent Corticotroph Pituitary Carcinoma. World neurosurgery. 2019 Feb;
[PubMed PMID: 30447452]