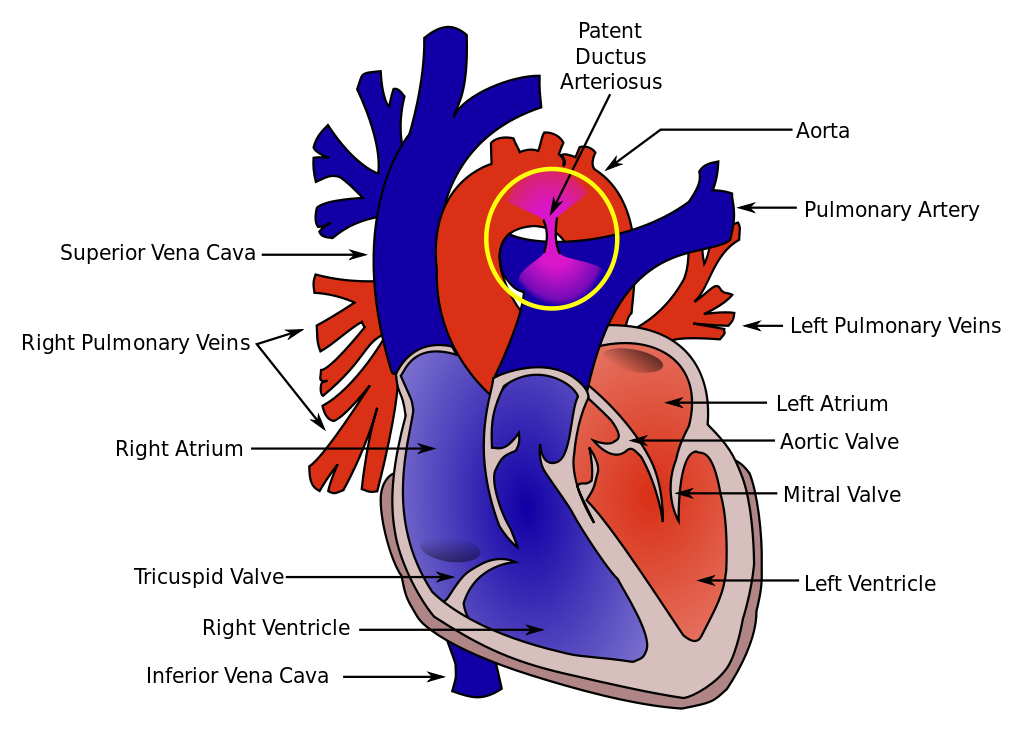
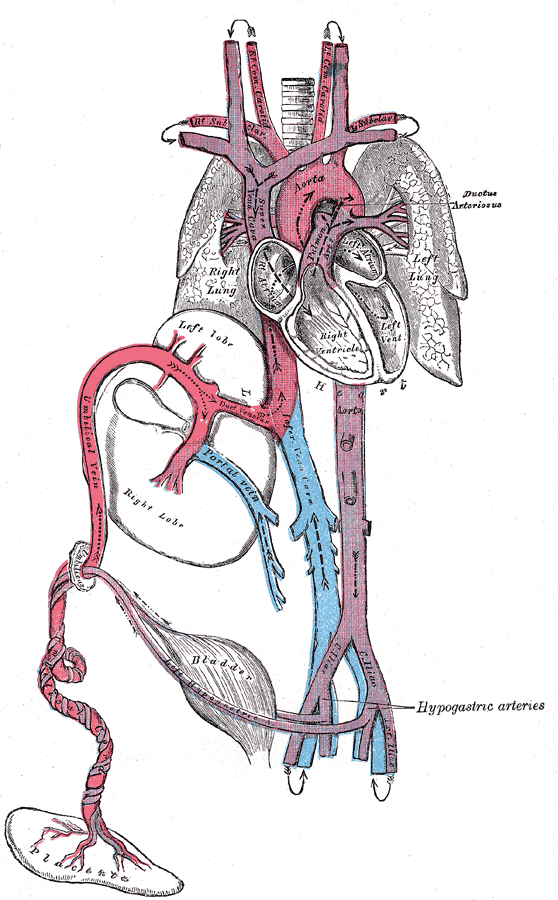
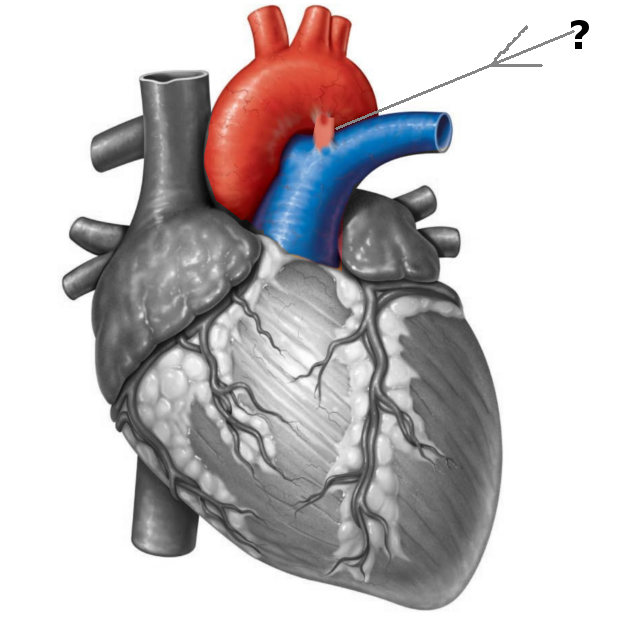
[1]
Fink D, Nitzan I, Bin-Nun A, Mimouni F, Hammerman C. Ductus arteriosus outcome with focus on the initially patent but hemodynamically insignificant ductus in preterm neonates. Journal of perinatology : official journal of the California Perinatal Association. 2018 Nov:38(11):1526-1531. doi: 10.1038/s41372-018-0204-x. Epub 2018 Aug 17
[PubMed PMID: 30120422]
[2]
Clyman RI. Patent ductus arteriosus, its treatments, and the risks of pulmonary morbidity. Seminars in perinatology. 2018 Jun:42(4):235-242. doi: 10.1053/j.semperi.2018.05.006. Epub 2018 May 10
[PubMed PMID: 29958703]
[3]
Jasani B, Weisz DE, McNamara PJ. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Seminars in perinatology. 2018 Jun:42(4):243-252. doi: 10.1053/j.semperi.2018.05.007. Epub 2018 May 24
[PubMed PMID: 29958702]
[4]
Hung YC, Yeh JL, Hsu JH. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. International journal of molecular sciences. 2018 Jun 25:19(7):. doi: 10.3390/ijms19071861. Epub 2018 Jun 25
[PubMed PMID: 29941785]
[5]
Reese J, Scott TA, Patrick SW. Changing patterns of patent ductus arteriosus surgical ligation in the United States. Seminars in perinatology. 2018 Jun:42(4):253-261. doi: 10.1053/j.semperi.2018.05.008. Epub 2018 May 22
[PubMed PMID: 29954594]
[6]
Iwashima S, Satake E, Uchiyama H, Seki K, Ishikawa T. Closure time of ductus arteriosus after birth based on survival analysis. Early human development. 2018 Jun:121():37-43. doi: 10.1016/j.earlhumdev.2018.05.003. Epub 2018 May 10
[PubMed PMID: 29754023]
[7]
P S,Jose J,George OK, Contemporary outcomes of percutaneous closure of patent ductus arteriosus in adolescents and adults. Indian heart journal. 2018 Mar - Apr
[PubMed PMID: 29716712]
[8]
Weisz DE, Giesinger RE. Surgical management of a patent ductus arteriosus: Is this still an option? Seminars in fetal & neonatal medicine. 2018 Aug:23(4):255-266. doi: 10.1016/j.siny.2018.03.003. Epub 2018 Mar 7
[PubMed PMID: 29636280]
[9]
de Boode WP, Kluckow M, McNamara PJ, Gupta S. Role of neonatologist-performed echocardiography in the assessment and management of patent ductus arteriosus physiology in the newborn. Seminars in fetal & neonatal medicine. 2018 Aug:23(4):292-297. doi: 10.1016/j.siny.2018.03.007. Epub 2018 Mar 7
[PubMed PMID: 29551482]
[10]
de Freitas Martins F, Ibarra Rios D, F Resende MH, Javed H, Weisz D, Jain A, de Andrade Lopes JM, McNamara PJ. Relationship of Patent Ductus Arteriosus Size to Echocardiographic Markers of Shunt Volume. The Journal of pediatrics. 2018 Nov:202():50-55.e3. doi: 10.1016/j.jpeds.2018.06.045. Epub 2018 Aug 13
[PubMed PMID: 30115452]
[11]
van Laere D,van Overmeire B,Gupta S,El-Khuffash A,Savoia M,McNamara PJ,Schwarz CE,de Boode WP,European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE)., Application of NPE in the assessment of a patent ductus arteriosus. Pediatric research. 2018 Jul
[PubMed PMID: 30072803]
[12]
Singh Y, Katheria A, Tissot C. Functional Echocardiography in the Neonatal Intensive Care Unit. Indian pediatrics. 2018 May 15:55(5):417-424
[PubMed PMID: 29845957]
[13]
Henry BM, Hsieh WC, Sanna B, Vikse J, Taterra D, Tomaszewski KA. Incidence, Risk Factors, and Comorbidities of Vocal Cord Paralysis After Surgical Closure of a Patent Ductus Arteriosus: A Meta-analysis. Pediatric cardiology. 2019 Jan:40(1):116-125. doi: 10.1007/s00246-018-1967-8. Epub 2018 Aug 24
[PubMed PMID: 30167748]
Level 1 (high-level) evidence
[14]
Halil H, Buyuktiryaki M, Atay FY, Oncel MY, Uras N. Reopening of the ductus arteriosus in preterm infants; Clinical aspects and subsequent consequences. Journal of neonatal-perinatal medicine. 2018:11(3):273-279. doi: 10.3233/NPM-17136. Epub
[PubMed PMID: 30149471]
[15]
Hundscheid T, Onland W, van Overmeire B, Dijk P, van Kaam AHLC, Dijkman KP, Kooi EMW, Villamor E, Kroon AA, Visser R, Vijlbrief DC, de Tollenaer SM, Cools F, van Laere D, Johansson AB, Hocq C, Zecic A, Adang E, Donders R, de Vries W, van Heijst AFJ, de Boode WP. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC pediatrics. 2018 Aug 4:18(1):262. doi: 10.1186/s12887-018-1215-7. Epub 2018 Aug 4
[PubMed PMID: 30077184]
Level 1 (high-level) evidence
[16]
Corcoran S, Briggs K, O' Connor H, Mullers S, Monteith C, Donnelly J, Dicker P, Franklin O, Malone FD, Breathnach FM. Prenatal detection of major congenital heart disease - optimising resources to improve outcomes. European journal of obstetrics, gynecology, and reproductive biology. 2016 Aug:203():260-3. doi: 10.1016/j.ejogrb.2016.06.008. Epub 2016 Jun 18
[PubMed PMID: 27359082]
[17]
Luca AC, Holoc AS, Iordache C. CONGENITAL HEART MALFORMATIONS IN NEWBORN BABIES WITH LOW BIRTH WEIGHT. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2015 Apr-Jun:119(2):353-60
[PubMed PMID: 26204636]
[18]
Whitfield K, Barkeij C, North A. MEDICATION MANAGEMENT OF THE EXTREMELY PREMATURE NEONATE - THE IMPACT OF A SPECIALIST PHARMACIST. Archives of disease in childhood. 2016 Sep:101(9):e2. doi: 10.1136/archdischild-2016-311535.55. Epub
[PubMed PMID: 27540237]
[19]
Aygün A, Poryo M, Wagenpfeil G, Wissing A, Ebrahimi-Fakhari D, Zemlin M, Gortner L, Meyer S. Birth weight, Apgar scores and gentamicin were associated with acute kidney injuries in VLBW neonates requiring treatment for patent ductus arteriosus. Acta paediatrica (Oslo, Norway : 1992). 2019 Apr:108(4):645-653. doi: 10.1111/apa.14563. Epub 2018 Oct 1
[PubMed PMID: 30178614]
[20]
Liu J, Gao L, Tan HL, Zheng QH, Liu L, Wang Z. Transcatheter closure through single venous approach for young children with patent ductus arteriosus: A retrospective study of 686 cases. Medicine. 2018 Aug:97(35):e11958. doi: 10.1097/MD.0000000000011958. Epub
[PubMed PMID: 30170394]
Level 2 (mid-level) evidence
[21]
Mashally S, Nield LE, McNamara PJ, Martins FF, El-Khuffash A, Jain A, Weisz DE. Late oral acetaminophen versus immediate surgical ligation in preterm infants with persistent large patent ductus arteriosus. The Journal of thoracic and cardiovascular surgery. 2018 Nov:156(5):1937-1944. doi: 10.1016/j.jtcvs.2018.05.098. Epub 2018 Jul 11
[PubMed PMID: 30007780]