Continuing Education Activity
In 2010, over 16 million people in the US required treatment for dysphagia, either in the community or an inpatient setting. It is estimated that nearly 50% of hospitalized patients have a swallowing disorder, exposing them to the risk of aspiration pneumonia (with concomitant increased length of hospital stay), increased re-hospitalization, and increased mortality. By identifying at-risk patients early on, appropriate management can commence, helping reduce the risk of aspiration. The videofluoroscopic swallow study is an important initial diagnostic study for dysphagia as it demonstrates the dynamic motion of the upper aerodigestive pathway in real-time with the motion of food bolus passing through it. This activity outlines and reviews the role of the healthcare team in managing patients who undergo a swallow study.
Objectives:
Identify the indications for a swallowing study.
Review the complications associated with a swallowing study.
Describe the typical imaging findings associated with an abnormal swallowing study.
Introduction
In 2010, over 16 million people in the US required treatment for dysphagia, either in the community or an inpatient setting. It is estimated that nearly 50% of hospitalized patients have a swallowing disorder, exposing them to the risk of aspiration pneumonia (with concomitant increased length of hospital stay), increased re-hospitalization, and increased mortality. By identifying at-risk patients early on, appropriate management can commence, helping reduce the risk of aspiration.[1]
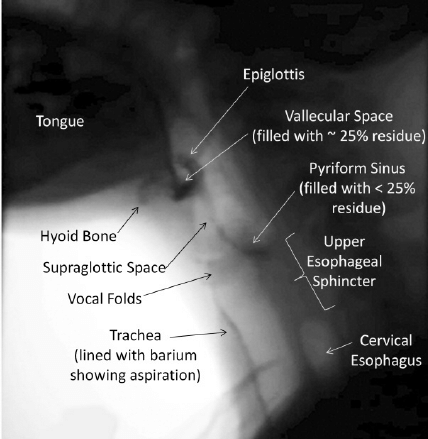
A holistic approach to nutritional needs can improve the patient’s overall quality of life, in addition to their psychological health. In elderly patients, difficulties with swallowing can cause anxiety around a meal, and panic associated with this activity of daily living can result in the individual socially isolating themselves. These can all contribute to chronic malnutrition and inanition. A videofluoroscopic swallow study is considered the gold standard diagnostic tool for the evaluation of dysphagia (see Image. Videofluoroscopic Swallow Study). It can assess dysphagia that may not be picked up by clinical examination or patient reporting and can be combined with Functional Endoscopic Evaluation of Swallowing (FEES) for a comprehensive evaluation of the swallowing mechanisms and mechanics.[2][3][4]
Procedures
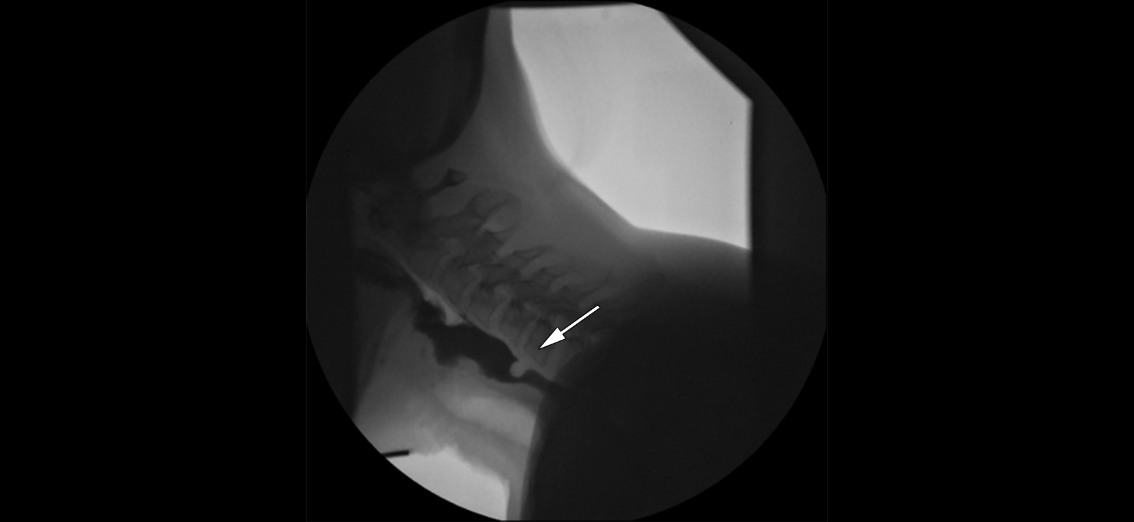
The patient is usually seated in the upright position on a chair or wheelchair for the duration of the examination to emulate the optimal physiological position for swallowing, with study views made in both the anterior-posterior and lateral views (see Image. Lateral Video Fluoroscopic Swallow Study). The field of study is from the oral cavity to the upper esophagus. Fluoroscopic images are recorded on a screen of the patient’s oral intake, allowing the healthcare professional to review the study retrospectively.
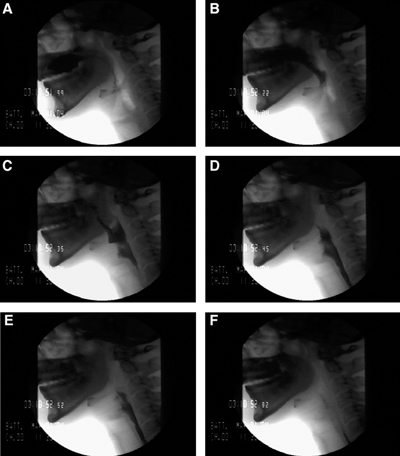
The clinician provides a selection of food and drink of different consistencies to chew and swallow under fluoroscopy. The selection of test food is based on the swallowing difficulty being investigated. The varying viscosities used are carefully selected by the speech and language therapist to account for the potential severity of dysphagia posed by the subject, as well as any known anatomic or functional abnormalities. These test foods/liquids are pre-mixed with barium as the contrasting agent, though water-soluble contrast agents such as gastrograffin can also be utilized. With the radio-opaque bolus, images are obtained at different stages of the swallowing process to highlight and analyze the cause of dysphagia (see Image. Stages of the Swallowing Process).[5]
Indications
A videofluoroscopic swallow study is most often used to evaluate:
- Potential aspiration
- Oropharyngeal dysphagia
- Odynophagia or globus sensation
Evaluations for dysphagia or aspiration are often performed:
- Post cerebral infarct
- Patient with a history of neuromuscular disease
- Following head and neck surgery
- After radiation to the head or neck
There are few absolute contraindications. If the patient is grossly aspirating on an exam, then the exam can be performed quickly and carefully to gather limited information on why the aspiration is occurring and which structures are involved, but this must always be balanced against the risk of large volume aspiration of barium contrast medium, which can markedly impair respiratory function.
The patient usually must be able to sit or stand, and the exam is only rarely performed in a supine position. The patient must be coherent and able to follow instructions to participate in the examination.[6]
Potential Diagnosis
For infants, potential causes of dysphagia include premature birth, congenital hiatal hernia, bronchopulmonary dysplasia, oesophageal atresia, trachea-oesophageal fistulas, congenital heart disease, gastro-oesophageal reflux, and neurological disorders[7].
Oropharyngeal cancers (more common in the 5th and 6th decades of life) can cause dysphagia via obstruction by the primary tumor or by the reduced movement of the mandible or tongue. Management options for oropharyngeal cancer are also known to cause dysphagia- surgical resection and subsequent reconstruction (for example, the base of tongue resection) removes the normal anatomy involved in moving food boluses and reconstructs it with immobile or hypo-mobile tissue. Radiotherapy of the head and neck region causes physiological changes (e.g., mucositis, hyposalivation, and tissue fibrosis) that impair normal bolus formation and propulsion mechanisms[8].
Neurological causes of dysphagia can occur acutely, for example, in stroke or traumatic brain injury. Conversely, they can progress more slowly over time as part of neurodegenerative conditions such as Parkinson disease.
Approximately 780,000 individuals in the US have a stroke in any given year, with aspiration pneumonia affecting one-third of stroke sufferers[9]. This is most often a result of abnormal/absent swallow reflex, inefficient lip closure, and limited tongue movement due to stroke-related cranial neuropathies. Therefore, it is imperative to provide nutrition via a tube for the first few weeks of a recent stroke if the patient cannot maintain adequate oral intake.[10]
With traumatic brain injuries, a common cause for dysphagia is the motor pattern reorganization following injury. This may be further prolonged by vocal cord paralysis following a long period of endotracheal tube intubation while in intensive care.
An estimated 80% of patients diagnosed with Parkinson disease have some degree of dysphagia (caused by both dopaminergic and non-dopaminergic disease pathways).[11] Aspiration pneumonia is a leading cause of death in Parkinson disease. Often patients may not clinically demonstrate signs of dysphagia, but it is picked up on the videofluoroscopic swallow study.
Iatrogenic causes include endotracheal intubation (which can cause damage to the recurrent laryngeal nerve supplying the vocal cord) and surgeries involving the head and neck or cardiothoracic surgery.
With aging, abnormalities in the cough reflex may develop as a result of the reduction of sensitivity in both the pharynx and larynx. Coupled with a delay in the initiation of the swallowing reflex and the slowing of food boluses passing along the oral and pharyngeal stages of swallowing, illness can cause the elderly to be more prone to dysphagia and aspiration pneumonia. An estimated 13% of nursing home residents are deemed to have some degree of dysphagia. Carer providers need to liaise with speech and language therapists to identify individuals who are considered vulnerable.
Other causes of dysphagia include:
38% of patients with multiple sclerosis, ALS (80% of patients are affected by the advanced stages of this disease. One in three patients demonstrates dysphagia from bulbar palsy.[12][13] Cranial nerves IX, X, and XII are affected), muscular dystrophy, myasthenia gravis, dementia, and spinal cord injuries.[14][15][16]
Normal and Critical Findings
To be able to interpret videofluoroscopic swallow studies, it is important to appreciate the stages of a normal swallow. These can be divided into oral preparatory, oral, pharyngeal, and oesophageal stages as follows:
The bolus is placed on the anterior mouth floor or against the hard palate for liquids. Initially, a seal is formed by the soft palate and posterior tongue to prevent liquid from entering the oropharynx. The tongue tip is then raised to the hard palate, propelling the liquid bolus into the pharynx, with the posterior tongue lowering to open access into the oropharynx. Food boluses are manipulated by the tongue backward along the palate while the jaw opens to allow the bolus to enter the pharynx. It arrives at the valleculae for the pharyngeal stage.
With the pharyngeal stage of swallowing, the nasopharynx becomes separated through soft palate elevation- this prevents food regurgitation into the nasopharynx. At the same time, the base of the tongue retracts to manipulate the bolus against the pharyngeal wall. The pharyngeal constrictors contract from superior to inferior, propelling the food bolus downwards towards the esophagus. The pharyngeal stage has adaptations to help protect the airway from aspiration- the epiglottis moves to seal close the laryngeal vestibule, while the vocal folds close together to seal the glottis. At this point, the upper oesophageal sphincter opens by suprahyoid and thyrohyoid muscle contraction to allow entry into the esophagus.
At the oesophageal stage of swallowing, the bolus is propelled through the entire length of the esophagus by peristalsis. There is an initial relaxation of the muscle around the bolus, followed by muscle contraction that tracks the food bolus down past the lower oesophageal sphincter and into the stomach. Note the lower two-thirds of the esophagus is smooth muscle, with the autonomic nervous system controlling peristalsis, which is involuntary.[17]
The swallow is initially assessed in the lateral plane. This is to view the passage of the bolus in its entirety into the esophagus. Nasopharyngeal regurgitation, the elevation of the hyoid bone, tilting and sealing by the epiglottis, residual food in either the pyriform sinuses or valleculae, and abnormal upper oesophageal sphincter opening are all standard endpoints for assessment. An additional benefit of the lateral plane is the ability to assess for the entry/penetration of test boluses into the airway passages (larynx) and to determine at what stage this may occur. The anterior-posterior plane is then examined, which provides the added benefit of determining asymmetry if pathology is present. Videofluoroscopy allows the calculation of the Oral Transit Time and Pharyngeal Transit Time to calculate the Oropharyngeal Swallowing Efficiency. The severity of aspiration (penetration) using the 8-point Penetration-Aspiration Scale (PAS), with 1 meaning the absence of penetration of contrast below the vocal cords.[18]
Poor oral control can be demonstrated in videofluoroscopy by material falling through the lips (e.g., neuropathies), the inability of the subject to form a bolus using their tongue and to subsequently correctly position it in the oral cavity (e.g., stroke), or by a lack of seal formation (posterior tongue not adequately contacting the soft palate) leading to leakage of material and possible laryngeal penetration and aspiration. Poor tongue coordination, or hesitant initiation of swallowing, can show stasis of material within the oral cavity. Failure of soft palate elevation during swallowing subsequently leads to an insufficient palatopharyngeal seal, resulting in nasopharyngeal regurgitation.
Critical findings demonstrated during laryngeal elevation include decreased hyoid bone elevation (it normally elevates to appose the angle of the mandible) and reduced epiglottis movement. The latter suggests impaired glottic protection with a significant risk of material entering the supraglottic region and leading to aspiration. Note that if contrast material enters the laryngeal vestibule, this is termed laryngeal penetration, while contrast entering below the vocal cords is termed aspiration. Material may be seen to remain in the valleculae or piriform sinuses after swallowing (pooling) - this is observed in cerebrovascular accidents and sometimes after radiation therapy[19].
Cricopharyngeal prominence is observed with advanced age, oesophageal motility disorders, Zenker diverticulum, and cricopharyngeal achalasia[20]. It leads to the narrowing of the oesophageal lumen, therefore causing dysphagia. A Zenker diverticulum (posterior pharyngeal pouch) presents itself at Killian's dehiscence- a point of anatomical weakness between the cricopharyngeus and thyropharyngeus. Contrast material is regurgitated from the diverticulum post-swallow and can lead to overflow aspiration. The diverticular pouch is often typically visible on an anteroposterior view as well.[21]
Complications
Videofluoroscopic swallow study uses x-rays for image study. Due to the use of ionizing radiation, there is a recognized risk of cellular damage and potential cancer induction. The risk becomes higher with greater radiation doses used.
Some individuals may have allergies to the contrast material, and it is important to obtain consent from the patient. Alternate materials, such as gastrograffin, may be used in such situations. As with other radiological investigations, pregnancy status needs to be determined before commencing the study.
As the patient is being assessed for dysphagia, the radio-opaque test boluses may enter the airway passages- i.e., aspiration. This risk is reduced when the speech and language pathologist/therapist commences the study using a material with the easiest consistency for the patient to swallow before escalating to more difficult consistencies.
Clinical Significance
Overall, dysphagia increases the morbidity and mortality of patients. Therefore, dysphagia must be diagnosed and managed early to maintain the best possible quality of life for the individuals concerned. By making a prompt diagnosis and identifying the cause, healthcare professionals can personalize management to the individual patient.
Speech and language therapists will determine the abnormal stage(s) of a swallow and can make suggestions to the clinical team/carers/patient themselves. Management plans can include: advising that the patient becomes ‘Nil by Mouth’ and fed via a tube, e.g., nasogastric tubes or percutaneous endoscopic gastrostomy, requesting the patient avoid food of certain consistencies, e.g., ‘soft diet only’ or using maneuvers when eating.
Interprofessional healthcare team members need to keep up to date with the latest guidelines and skillsets required to manage patients presenting with swallowing disorders. The American Board of Swallowing and Swallowing Disorders provides specialty certification. Speech and language pathologists/therapists have expertise in assessing, diagnosing, and managing individuals with dysphagia and work inter-professionally with dieticians, radiologists, nurses, doctors, and carers to improve the quality of life and minimize risk.[4]