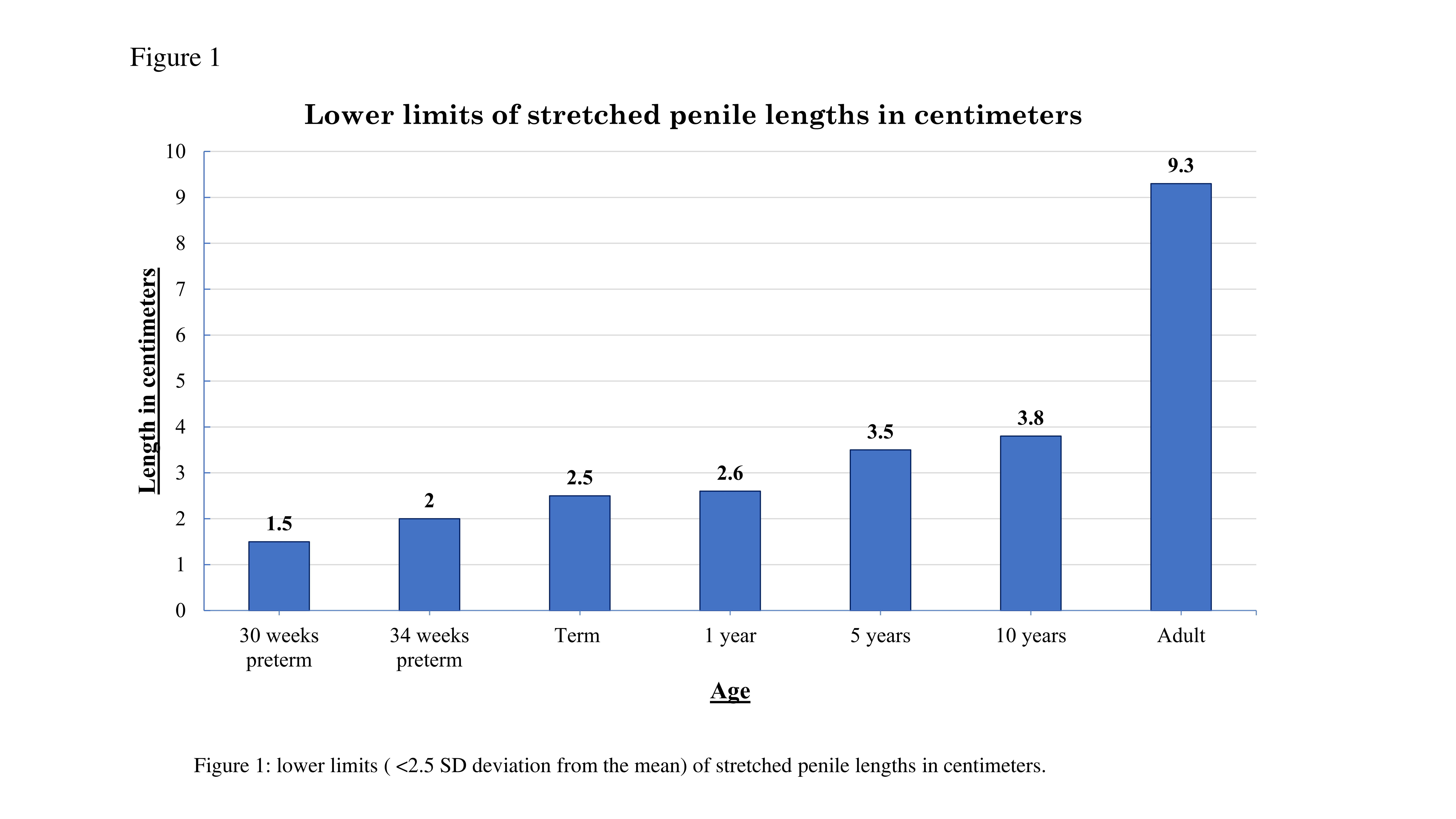
[1]
Johnson P, Maxwell D. Fetal penile length. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 Apr:15(4):308-10
[PubMed PMID: 10895450]
[2]
Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. The Journal of clinical endocrinology and metabolism. 2005 May:90(5):3122-7
[PubMed PMID: 15728198]
[3]
Hatipoğlu N, Kurtoğlu S. Micropenis: etiology, diagnosis and treatment approaches. Journal of clinical research in pediatric endocrinology. 2013:5(4):217-23. doi: 10.4274/Jcrpe.1135. Epub
[PubMed PMID: 24379029]
[4]
Nelson CP, Park JM, Wan J, Bloom DA, Dunn RL, Wei JT. The increasing incidence of congenital penile anomalies in the United States. The Journal of urology. 2005 Oct:174(4 Pt 2):1573-6
[PubMed PMID: 16148654]
[5]
Cheng PK, Chanoine JP. Should the definition of micropenis vary according to ethnicity? Hormone research. 2001:55(6):278-81
[PubMed PMID: 11805431]
[6]
Gabrich PN, Vasconcelos JS, Damião R, Silva EA. Penile anthropometry in Brazilian children and adolescents. Jornal de pediatria. 2007 Sep-Oct:83(5):441-6. doi: 10.2223/JPED.1671. Epub 2007 Aug 3
[PubMed PMID: 17676248]
[7]
Matsuo N, Ishii T, Takayama JI, Miwa M, Hasegawa T. Reference standard of penile size and prevalence of buried penis in Japanese newborn male infants. Endocrine journal. 2014:61(9):849-53
[PubMed PMID: 24931740]
[8]
Cinaz P, Yeşilkaya E, Onganlar YH, Boyraz M, Bideci A, Camurdan O, Karaoğlu AB. Penile anthropometry of normal prepubertal boys in Turkey. Acta paediatrica (Oslo, Norway : 1992). 2012 Jan:101(1):e33-6. doi: 10.1111/j.1651-2227.2011.02386.x. Epub 2011 Oct 4
[PubMed PMID: 21682766]
[9]
Sultan C, Paris F, Jeandel C, Lumbroso S, Galifer RB. Ambiguous genitalia in the newborn. Seminars in reproductive medicine. 2002 Aug:20(3):181-8
[PubMed PMID: 12428198]
[10]
Guthrie RD, Smith DW, Graham CB. Testosterone treatment for micropenis during early childhood. The Journal of pediatrics. 1973 Aug:83(2):247-52
[PubMed PMID: 4717581]
[11]
Arisaka O, Hoshi M, Kanazawa S, Nakajima D, Numata M, Nishikura K, Oyama M, Nitta A, Kuribayashi T, Kano K, Nakayama Y, Yamashiro Y. Systemic effects of transdermal testosterone for the treatment of microphallus in children. Pediatrics international : official journal of the Japan Pediatric Society. 2001 Apr:43(2):134-6
[PubMed PMID: 11285063]
[12]
Ong YC, Wong HB, Adaikan G, Yong EL. Directed pharmacological therapy of ambiguous genitalia due to an androgen receptor gene mutation. Lancet (London, England). 1999 Oct 23:354(9188):1444-5
[PubMed PMID: 10543676]
[13]
Bertelloni S, Scaramuzzo RT, Parrini D, Baldinotti F, Tumini S, Ghirri P. Early diagnosis of 5alpha-reductase deficiency in newborns. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2007:1(3):147-51. doi: 10.1159/000102103. Epub
[PubMed PMID: 18391525]
[14]
Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. The Journal of clinical endocrinology and metabolism. 2008 Jun:93(6):2202-5. doi: 10.1210/jc.2008-0121. Epub 2008 Apr 1
[PubMed PMID: 18381569]