Continuing Education Activity
The genus Nocardia is an aerobic actinomycete, catalase-positive, gram-positive bacillus, with a branching filamentous form that can cause pulmonary infection (most common), primary cutaneous infection and also dissemination to other sites. Typically, Nocardia is an opportunistic pathogen that primarily affects immunosuppressed patients. This activity reviews the evaluation and treatment of Nocardia infection and highlights the role of the interprofessional team in the care of patients with this condition.
Objectives:
- Identify patients at high risk of developing nocardiosis.
- Summarize the clinical presentations of nocardiosis based upon the infection site.
- Outline the treatment and management options available for nocardiosis.
- Review interprofessional team strategies for improving care coordination and communication to advance nocardiosis treatment and improve outcomes.
Introduction
The genus Nocardia is an aerobic actinomycete, catalase-positive, gram-positive bacillus, with a branching filamentous form first described in 1888 by Edmond Nocard.[1][2] Nocardia sp. is found worldwide in a myriad of environments.[3] Disease resulting from Nocardia infection results in a variety of symptoms based upon the location of the infection. The classic sites of infection include pulmonary infection (most common) and primary cutaneous infection; however, dissemination to other sites and CNS involvement often occurs.[2][4] Typically, Nocardia is an opportunistic pathogen that primarily affects immunosuppressed patients.[2][3] However, Nocardia can also infect immunocompetent individuals, especially the cutaneous form of the disease.
Etiology
Nocardia has been reported to be found ubiquitously in the environment and has been cultured from both fresh and saltwater, dust, soil, decaying vegetation, and decaying organic matter.[2][5][6] The majority of Nocardia infections are thought to be due to inhalation of bacteria via environmental sources (i.e., dust), leading to the most common form of the infection, pulmonary nocardiosis.[5]
Contrastingly, cutaneous nocardiosis arises via the inoculation of traumatic skin lesions. Other methods of infection that have been implicated include hospitalized acquired infections where clusters of nocardiosis have been described, and infection is transmitted from central venous catheters.[7][8] Once a patient has a Nocardia infection, whether symptomatic or asymptomatic, it can spread hematogenously or by local extensions. Overall, immunocompromised individuals are more susceptible to nocardiosis and disseminated spread.
Epidemiology
The CDC reports the incidence of nocardiosis in the US as 500-1000 cases per year. Globally, the incidence of Nocardia infection is not well known. The incidence of nocardiosis is thought to be increasing due to an increasing population and an increased number of immunocompromised individuals worldwide.[1][9][10] Nocardiosis affects all ages and races but is more common in males than females in a 3 to 1 ratio. Nocardia typically manifests as an opportunistic infection in immunocompromised patients, with 60% of patients with nocardiosis having immunosuppressive conditions.[2][6][11] However, up to 33% of patients are immunocompetent; this is especially true of cutaneous infections where immunocompetent individuals are the majority of cases.[3][5][12] Dissemination of both cutaneous and pulmonary nocardiosis is more likely in immunocompromised hosts.
Immunocompromised conditions associated with Nocardia infections include:
- HIV (most common underlying condition)[13][14]
- Cancer
- Chemotherapy treatment
- Corticosteroid treatment (present in more than half of patients with Nocardia infection according to one report)[13][14]
- Solid organ transplant recipients (especially lung transplant)[15]
- Allogenic hematopoietic stem cell transplant recipients[16]
- Diabetes
- Autoimmune diseases
- Chronic lung diseases such as COPD (many patients on corticosteroid therapy)[17]
- Any condition that results in deficient cell-mediated immunity
Pathophysiology
Found commonly in soil, presentations of Nocardia are:
- Primary cutaneous nocardiosis
- Cellulitic and sporotrichoid forms
- Actinomycetoma
- Disseminated disease (most common secondary to pulmonary involvement)
The most common form of Nocardia infection is pulmonary, and most common causing human infection are:
- N. asteroides
- N. brasiliensis
- N. caviae
Nocardia sp. is a member of the family Nocardiaceae (along with the genus Rhodococcus), belonging to the “aerobic actinomycetes” suborder. Currently, the genus Nocardia contains 80-100 species, of these, more than fifty have been implicated as human or animal pathogens.[3][18] Historically, Nocardia asteroides was implicated as the most common cause of numerous Nocardial human infections. However, it was later found to be a group of bacteria that have been further classified into separate species.[3][5] Various Nocardia species have been shown to be more prevalent in specific geographic locations. For example, Nocardia brasiliensis, a species associated with cutaneous infections (80% of all cutaneous Nocardia infection), is found more frequently in tropical and sub-tropical climates.[2] Nocardia infections vary significantly in symptomatology based on the site of infection. However, Nocardia typically produces suppurative necrosis with frequent abscess formation at most sites of infection, including pulmonary, cutaneous, and CNS. That being said, Nocardia also can form cavitary lesions, skin nodules, and cellulitis making diagnosis difficult as it is similar to many other bacterial and fungal infections.
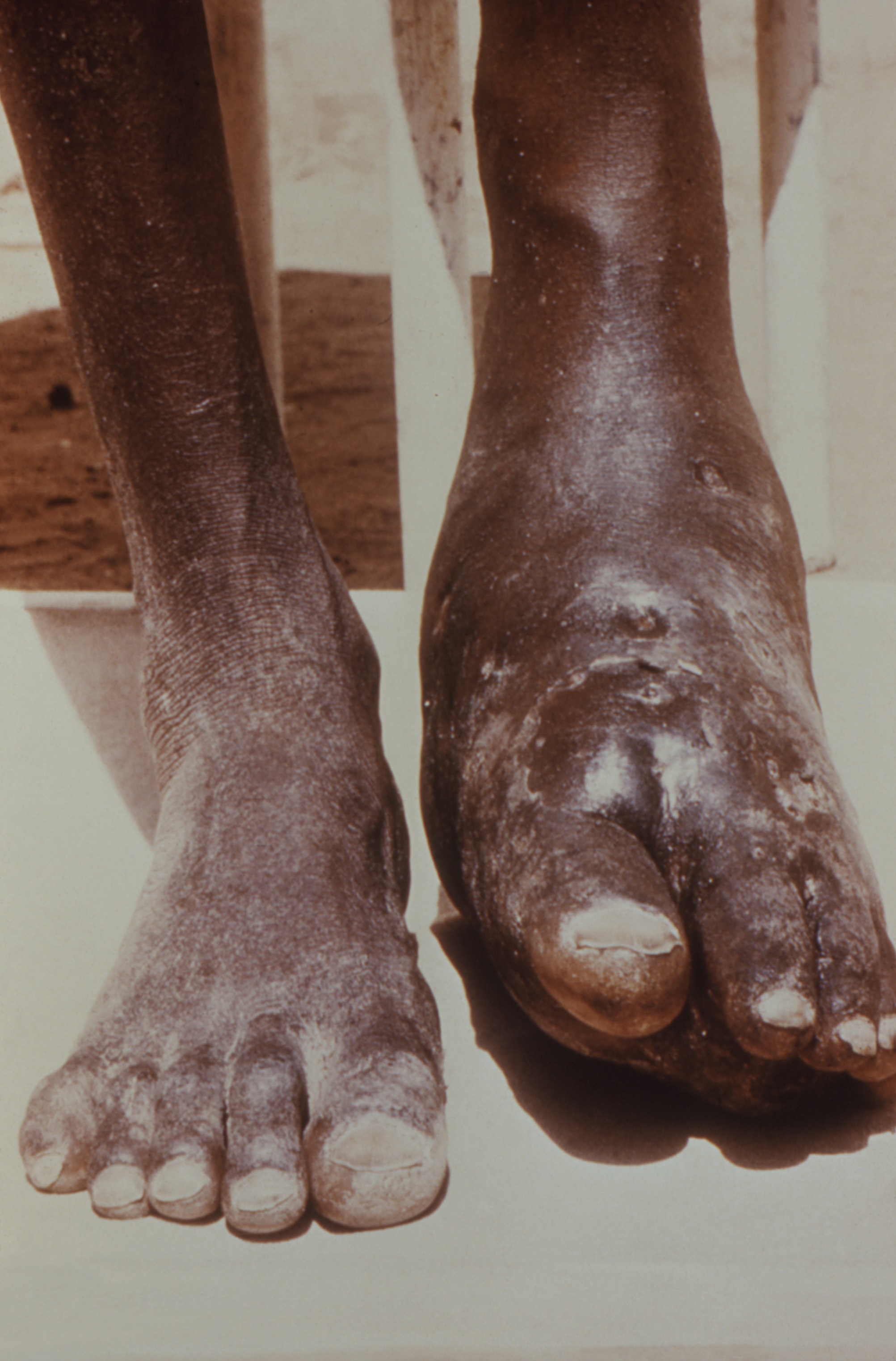
Primary cutaneous nocardiosis is most commonly caused by N. brasiliensis. Cutaneous nocardiosis from N. asteroides is usually secondary to hematogenous dissemination. Primary cutaneous nocardiosis is divided into three entities:
- Lymphocutaneous infection (often misdiagnosed for sporotrichosis)
- Mycetoma
- Superficial skin infection (ulceration, abscess, cellulitis)
Histopathology
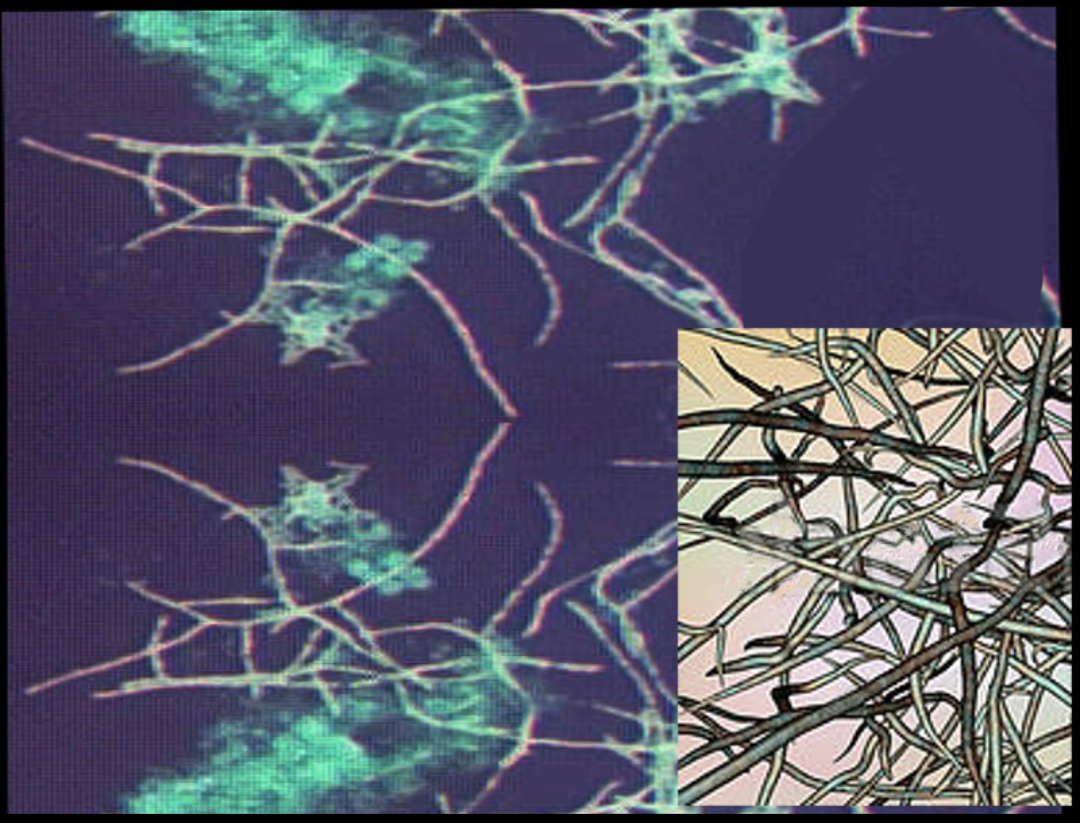
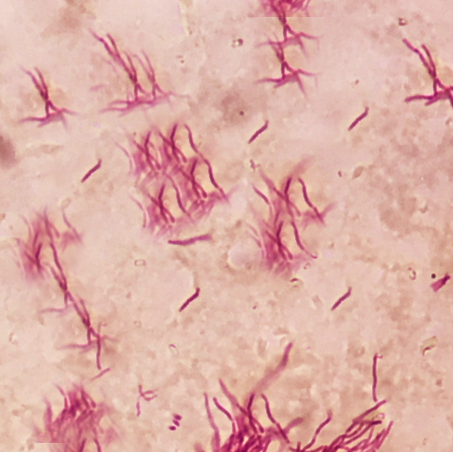
The characteristic morphology of Nocardia is a branching, filamentous, gram-positive rod when observed under the microscope. Nocardia can resemble Actinomyces species on gram stain. However, Nocardia is an obligate aerobe that stains weakly positive on acid-fast staining.[1]
Nocardia is typically weakly acid-fast after traditional carbol-fuchsin staining and positive on modified acid-fast staining due to the presence of mycolic acid in the cell wall. Unlike mycobacteria (also acid-fast positive), Nocardia has a “beaded” appearance on acid-fast staining.[3] Nocardia is catalase-positive and urease positive.
Toxicokinetics
Nocardia has low virulence, and so clinically significant disease occurs in patients with immunosuppression or weak immune systems. The virulence factors of Nocardia are the enzymes catalase and superoxide dismutase, which inactivates reactive oxygen species toxic to the bacteria produced by neutrophils.[2] Thus, patients with chronic granulomatous disease are susceptible to Nocardia infections. Nocardia also has “cord factor” similar to Mycobacterium tuberculosis preventing the fusion of phagosomes with lysosomes.[19][20]
History and Physical
The symptoms of nocardiosis vary depending on where the site of infection is. Patients with pulmonary nocardiosis can present with fever, weight loss, night sweats, cough, and chest pain. If the infection spreads to the CNS, symptoms can include headache, weakness, confusion, and seizures. Cutaneous nocardiosis can present with skin ulcers, nodules that are sometimes draining, and the infection can spread to the lymph nodes.
Delay in the diagnosis of an immunosuppressed patient with nocardiosis has been implicated in treatment failure and poor prognosis. Therefore, if there is suspicion for infection with Nocardia, it is imperative to notify the laboratory of this suspicion so that steps can be taken by the laboratory to identify the species and antibiotic sensitivity pattern.
Evaluation
Nocardiosis is a challenge to diagnose due to its non-specific findings and imaging that can suggest many different diagnoses (see DDX section). The diagnosis of nocardiosis is established with a culture of the causative organism from the infection site. Common culture samples include sputum, skin biopsy, abscess aspirates, or a sample from bronchoalveolar lavage. It can take several days (3-5 days) to isolate Nocardia in culture. Blood cultures for Nocardemia should be drawn in instances of pulmonary or disseminated disease, although Nocardia bacteremia is very rare.[21]
Imaging via computed tomography (CT) or chest radiograph is a valuable diagnostic tool, especially in evaluating pulmonary nocardiosis. However, findings vary significantly and can include irregular nodules (can be cavitary), diffuse pulmonary infiltrates, lung abscesses, or pleural effusion. Imaging findings help support a history and physical exam that may be concerning for nocardiosis.
All patients with nocardiosis (except those with mycetoma) should undergo brain CT or magnetic resonance imaging (MRI). If the primary infection spreads, the CNS is the most common location (44% of the time).[6] An intracranial abscess is the most common abnormality found on imaging. If concerned for nocardial meningitis, a lumbar puncture should be performed, and CNS analysis conducted. Nocardial meningitis is rare but presents similar to other bacterial meningitis.[22][23]
It is important to notify the laboratory when suspecting Nocardia as a possible infection. This will allow for gram staining and modified acid-fast staining helping to confirm diagnosis as soon as possible. This is especially important as it can take several days to culture Nocardia species. Similarly, this will help aid in initial treatment prior to obtaining antibiotic susceptibility patterns. The recent development of more rapid and precise PCR and 16S rDNA sequencing to identify Nocardia species may allow for faster diagnosis and treatment of nocardiosis.[24]
Treatment / Management
Since the 1940s, the mainstay treatment for Nocardia infections has been sulfonamides, the most common of which in the U.S. is trimethoprim-sulfamethoxazole (TMP-SMX).[2][5][25][26] Resistance to TMP-SMX as a single agent has been shown to be species-dependent with N. farcinica, N. nova, and N. otitidiscaviarum showing increased multi-drug resistance.[3][26] Due to this resistance, most clinicians now administer combination drug therapy, especially for patients with a serious infection, disseminated disease, or CNS involvement. The most common regiment includes TMP-SMX, amikacin, and imipenem. However, if the patient has CNS involvement, it is important to pick agents with good CNS penetration, such as TMP-SMX and ceftriaxone.[3][27] More recently, the oxazolidinone, linezolid, has been shown to have excellent coverage against all known pathogenic Nocardia species.[3][5]
Other agents to consider include:
- Carbapenems: imipenem, meropenem, or ertapenem, with imipenem having the best efficacy
- Quinolones: especially moxifloxacin
- Tetracyclines: minocycline
The duration of treatment is dependent on the location of infection and the immune status of the affected individual. For patients with pulmonary or multifocal disease that does not involve the CNS, a treatment regimen for 6-12 months is indicated. If the patient is immunosuppressed or has CNS involvement, they should be treated for a minimum of 12 months.[3]
Prophylaxis: Patients shown to be at greatest risk for Nocardia infection have been documented to be patients that are HIV positive.[13][14] Commonly, TMP-SMX is used as prophylaxis in HIV patients for coverage against Pneumocystis jiroveci pneumonia (PJP) or Toxoplasma gondii. Fortunately, this prophylaxis also has been shown to help reduce Nocardia infections.
Differential Diagnosis
Nocardia is extremely difficult to differentiate from other infectious etiologies. The differential diagnosis for primary sites of infection are outlined below:[3][10]
Pulmonary disease
- Fungal infections – aspergillosis, mucormycosis, histoplasmosis, blastomycosis, and cryptococcosis
- Actinomyces infection
- Mycobacterial infections – including Mycobacterium tuberculosis
- Lung malignancy
Cutaneous disease
- Superficial cellulitis – group A strep (GAS), staph aureus
- Lymphocutaneous – sporotrichosis, Mycobacterium marinum
- Mycetoma (late-stage) – actinomycosis, fungal infections
- Other: cutaneous leishmaniasis, Cryptococcus
CNS disease
- Malignancy
- Bacterial abscess
- Vascular infarction
- Other: toxoplasmosis, mycobacterial tuberculosis, fungal infection, cysticercosis
Prognosis
The morbidity and mortality of Nocardia depend on the site and extent of infection as well as underlying host factors. Prognosis varies based on the site of infection.[28]
- Skin and soft tissue infections: approximately 100% cured
- Pleuropulmonary infections: approximately 80% cured
- In the 1940s, pulmonary nocardiosis had a fatality rate of 100%, but with the advent of sulfonamide antibiotics and more recently combined antibiotic therapy, the mortality has decreased dramatically.[5] Currently, mortality rates of pulmonary nocardiosis are anywhere from 14% to 40%.[10]
- Disseminated nocardiosis: 3% cured
- CNS involvement: 50% to 85% cure rate[29]
Complications
Complications can arise due to the spread from the primary site of infection. This is especially true of pulmonary nocardiosis that has spread locally. This can result in pericarditis and superior vena cava (SVC) syndrome depending upon the location and extent of spread. Additionally, the risk of spread to the CNS results in a multitude of possible complications, including focal neurologic deficits and meningitis. Delay in the diagnosis of an immunosuppressed patient with nocardiosis has been implicated in treatment failure and poor prognosis.[30] Therefore, if there is suspicion for infection with Nocardia, it is imperative to notify the laboratory so that steps can be taken by the laboratory to identify the species and antibiotic sensitivity pattern as quickly as possible. Additionally, the advent of PCR and 16S rDNA sequencing to identify Nocardia species may allow for faster identification and sequencing.[24]
Deterrence and Patient Education
Overall, Nocardia is a ubiquitous bacteria that can result in many forms of infection. Infection is more likely in immunocompromised individuals, especially the pulmonary and CNS forms of nocardiosis. Patient's diagnosed with HIV should be aware of the various presentations of Nocardia. TMP-SMX prophylaxis can be given for individuals that are at increased risk for nocardial infection, although there are many strains that are resistant to TMP-SMX as a single agent.
While immunocompromised individuals are at greatest risk, immunocompetent individuals are also at risk for infection, particularly the cutaneous form of the infection. The main etiology of infection in these patients is due to a skin lesion that was contaminated with organic matter or soil. Therefore, a key way to lower the risk of cutaneous nocardiosis is to wear closed shoes and gloves when working in hazardous areas (construction sites, gardens, etc.). If a skin lesion is sustained, it is important to clean the wound thoroughly. If the area becomes red, swollen, and warm or if there is purulent drainage, a primary care clinician should be consulted.
In general, Nocardia infections are very rare but should be considered if the workup is negative for other common causes of the presenting symptoms.
Enhancing Healthcare Team Outcomes
Nocardial infection often poses a diagnostic dilemma. Nocardiosis exhibits signs and symptoms that vary greatly based upon the location of the infection (cutaneous, pulmonary, CNS, disseminated). Additionally, there is a myriad of differential diagnoses that present similarly to nocardial infection and are often more commonly occurring, including fungal and other bacterial etiologies. The history and physical exam are important in assessing a patient's risk for nocardiosis. Imaging may allow the clinician to rule down their differential diagnosis honing in on Nocardia as a potential source of the infection. The most important diagnostic step is obtaining a sample of the infection (skin biopsy, sputum sample, abscess aspirate) in order to run a gram-stain, acid-fast stain, and culture of the organism. Finally, newer PCR and DNA sequencing methods may allow for a faster species-specific diagnosis.[24]
If the diagnosis of nocardiosis is confirmed, it is important to look for dissemination from the primary source of infection. Patients with Nocardia infections are best managed by an interprofessional team. Radiology should be consulted for further workup, which includes brain imaging. Radiologists play an important role as 44% of the time disseminated pulmonary infections are found to have CNS involvement. Brain imaging may reveal one or more cerebral abscesses consistent with CNS nocardiosis.[6] Finally, infectious disease should be consulted. Due to the increasing resistance of Nocardia to TMP-SMX and other sulfonamides, it is important to consult infectious disease specialists for the best combination of antibiotics for the patient.
Since Nocardia infections are very rare, there is limited knowledge regarding the outcomes of Nocardia treatment. The outcomes of nocardial infections depend on the site of infection and the immune status of the host. However, to improve outcomes, prompt consultation with an interprofessional group of specialists is recommended so that appropriate treatment can be started.