Continuing Education Activity
Marcus Gunn pupil is indicative of a defect in the afferent pathway of the light reflex. To avoid the high morbidity associated with this condition, it must be promptly diagnosed and the cause should be treated. This activity reviews the evaluation of Marcus Gunn pupil and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
Objectives:
- Describe the etiology, epidemiology, and pathogenesis of Marcus Gunn pupil.
- Summarize the relevant history and physical examination of patients with Marcus Gunn pupil.
- Review the evaluation process of patients with Marcus Gunn pupil.
- Outline the prognosis and interprofessional care of patients with Marcus Gunn pupil.
Introduction
Marcus Gunn pupil (MGP) is the term given to an abnormal pupil showing aberrant pupillary response in certain ocular disorders. In literature, the term is often used synonymously with Marcus Gunn phenomenon or relative afferent pupillary defect (RAPD).[1] After exposure to bright light, a normal pupil constricts.[2]A Marcus Gunn pupil, on the other hand, has a relative weakness of the afferent limb of the pupillary light reflex compared to the other eye because of which when light is rapidly transferred from the normal eye to the eye with MGP, the MGP dilates instead of constricting. It is named after Scottish Ophthalmologist Robert Marcus Gunn.[3] Hirschberg first noted this phenomenon in the case of unilateral retrobulbar optic neuritis.[3] The presence of a relative afferent pupil defect (RAPD) is the hallmark of a unilateral afferent sensory abnormality or bilateral asymmetric visual loss.
An afferent pupillary response is characterized by:[4]
- Constriction of pupils of both eyes when the light stimulus is applied to the normal eye
2. Dilatation of pupils of both eyes when the light stimulus is rapidly transferred from the normal eye (after brief light exposure to the normal eye) to the affected eye.
Etiology
The relative afferent pupillary defect is seen in various disorders (see pathophysiology). All the causes may not cause clinically appreciable RAPD.
The causes include:
Lesions of the Optic Nerve and Visual Pathway
- Lesions of the optic nerve[5]
- Lesions of the optic chiasm
- Lesions of optic tract[6][7]
- Lesions of pretectum[8]
- Glaucoma[1]
- Visual field defect[9]
Lesions of the Retina/Posterior Segment
- Retinal detachment[4][10]
- Central retinal vein occlusion (CRVO)[11]
- Central serous chorioretinopathy[12] (CSCR)- may not be clinically appreciable.
- Macular degeneration[13]
- Retinitis pigmentosa[14] (RP)
- Endophthalmitis
Induced RAPD of the Contralateral Eye
- Dense cataract[15] (see following discussion)
- Eye patching[16] of one eye
- Dark adaptation[16] of one eye
Miscellaneous
- Amblyopia[17]- may not be clinically appreciable.
- Anisocoria[18]
Lesions of the optic tract, pretectum, dense cataract, and eye patching/dark adaptation produce RAPD in the contralateral eye.[15][16] RAPD will be induced in the smaller pupil when anisocoria is greater than 2 mm.[18]
Epidemiology
There is no specific age and sex predilection for Marcus Gunn pupil in isolation. In a study conducted using binocular pupilometer, 42% of the normal population showed an RAPD between 0.08 and 0.22 log units, and 6% had an RAPD between 0.23 and 0.39 log units.[19] This occurrence of RAPD was justified due to the inaccuracy of measurement or synaptic asymmetries of the visual pathway.
An RAPD is noted in more than 90% of cases of acute unilateral cases of optic neuritis, 91% cases of ischemic central retinal vein occlusion (CRVO), more than 50% cases of retinal detachment involving the macula, and 23% cases of primary open-angle glaucoma (POAG).[5][4][11][5][20]
Pathophysiology
When a bright light is shone on one eye, its pupil constricts. This pupillary constriction is known as a direct light reflex. Simultaneously, the pupil of the other eye also constricts. This response in the contralateral eye is known as a consensual light reflex. The light reflex is mediated by retinal photoreceptors and subserved by four neurons.[21]
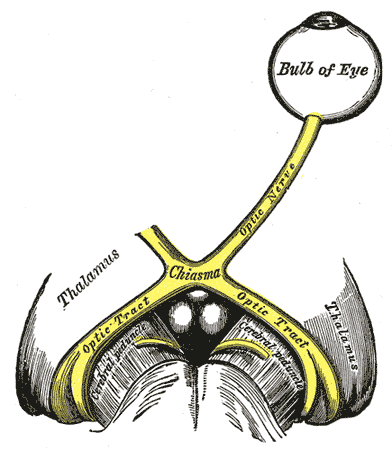
- First (sensory afferents) connects each retina with both pretectal nuclei located in midbrain at the level of the superior colliculus. Impulses from the nasal half of retina decussate in the optic chiasm and pass via contralateral optic tract to reach the contralateral pretectal nucleus. Impulses from the temporal half of retina travel via the ipsilateral optic tract to reach the ipsilateral pretectal nucleus. It is estimated that the fibers decussate within the chiasm in a ratio of crossed to uncrossed fibers of 53:47.[22] According to a new model, there may be a partial crossing of temporal fibers in the chiasm in addition to the crossing of nasal fibers, leading to larger input from one eye into the contralateral optic tract.[7]
- Second (internuncial) connects each pretectal nucleus to both Edinger-Westphal nuclei. Thus uniocular light stimulus evokes bilateral pupillary constriction. This consensual light reflex results in an almost similar amount of pupillary constriction since pupillomotor output to both the eyes is the same.[23]
- Third (preganglionic motor) connects the Edinger-Westphal nucleus to the ciliary ganglion. These fibers pass through the inferior division of the oculomotor nerve.
- Fourth (postganglionic motor) innervate sphincter pupillae via short ciliary nerves.
The relative afferent pupillary defect is usually seen in lesions of the afferent arm of the light pathway. When the light is shifted from the normal eye to abnormal eye, the total pupillomotor input is reduced, hence the amount of pupillary constriction is less when compared to stimulation of the normal eye.[24] This difference in pupillomotor input gives rise to relative afferent pupillary defect.
RAPD in Lesions of the Optic Nerve
Ischemic optic neuropathies, such as arteritic ischemic optic neuropathy (AION), Nonarteritic ischemic optic neuropathy (NAION) causes RAPD via optic nerve ischemia and infarction secondary to optic nerve edema. Other diseases causing such asymmetric or unilateral damage of optic nerve resulting in RAPD include glaucoma, optic neuritis, retrobulbar neuritis, and traumatic optic neuropathy.
RAPD in Lesions of the Optic Tract[6][7]
The axons of the retinal ganglion cells from the nasal retina decussate in the optic chiasm joining the temporal fibers of the other side. In addition, there may be a partial crossing of temporal fibers in the chiasm.[7] Thus there is an asymmetry of pupillomotor input into the optic tracts. There is larger input from one eye to the contralateral tract. Thus patients with unilateral lesions of the optic tract show a marked relative afferent pupillary defect in the eye contralateral to the lesion. It usually occurs in the eye with temporal field defect.
RAPD in Lesions of the Pretectum[8]
The asymmetry of pupillomotor input extends from the optic tract to the pretectal nucleus. Hence lesions of pretectum produce RAPD in the contralateral eye. The relative afferent pupillary defect in such situations is without the associated loss of visual acuity, color vision, or visual field. This is because just before fibers reach lateral geniculate body (light pathway), few fibers (pupillary reflex) go to the pretectum. Hence for the lesions at the brachium of the superior colliculus (just before the fibers reach the pretectal nucleus), vision is not affected.
RAPD in Glaucoma and Visual Field Defects[1][9]
In conditions such as glaucoma, RAPD occurs secondary to damage to retinal ganglion cells and loss of retinal nerve fiber layer.[25] At least 26% of retinal ganglion cells should be damaged to elicit an RAPD.[26] An RAPD will be noticed only in asymmetric severity of the glaucomatous damage. An RAPD may be noted even before the appearance of diagnostic glaucomatous visual field defects.[27] It was shown that central 30 degrees of the visual field is the most effective for pupillomotor input, and the contribution of the visual field asymmetry outside of 30 degrees to the relative afferent pupillary defect is clinically negligible.[9]
RAPD in Retinal Disorders[4][10][11][12][13][14]
RAPD occurs secondary to the death of photoreceptors and viable retina.[13] The central retina is the most effective for pupillomotor input.[28] The light stimulus to the central retina elicits a greater pupillary response than the same stimulus applied to the peripheral retina. The detachment of each peripheral quadrant of the retina causes 0.35 log units of a defect, and detachment of macula causes 0.68 log units of a defect.[10] However, this is only an estimate as the standard deviation was in the range of +/- 0.65 log units.[10] Ischemic CRVO usually produces 0.9 to 1.2 log units of a defect (usually more than 0.7 log units), and non-ischemic CRVO produces less than 0.6 log units of a defect.[11] A Macular disease does not usually cause RAPD unless it is very severe.[29] For a given RAPD, visual acuity is impaired to a far greater magnitude in macular disease, whereas, in optic neuropathy, vision loss may corroborate with the quantification of RAPD.[12] Localized macular dystrophies/degenerations and diffuse retinal dystrophies such as retinitis pigmentosa usually do not show an RAPD response unless the disease is asymmetric. Even in that case, the RAPD may be subclinical. However, variants such as unilateral retinitis pigmentosa can show an RAPD usually of <0.3 log units.[14] CSCR may cause mild RAPD (usually ≤ 0.4 log units, some cases may have RAPD up to 0.9 log units), which improves with the resolution of the subretinal fluid.[12]
Induced RAPD
RAPD in Cataract[15]
Detection of RAPD in the case of cataracts is controversial. RAPD in cataracts usually indicates underlying damage to the afferent visual pathway (damage of the optic nerve or the retina, or visual pathway) and not due to cataract in itself. Since the time of Galen, RAPD was noted to indicate poor visual gain post-cataract surgery.[3] A dense cataract causes an RAPD in the contralateral eye by increasing the pupillomotor effectiveness of the stimulus light likely due to increased intraocular light scattering by the cataract.[15] Peripheral photoreceptors may be stimulated by diffusely scattered light, and the light may bounce back again on the retina due to reflection by the posterior surface of the cataract.[15] Also, a dense cataract may induce dark adaptation of the affected eye.[15] A mature cataract has been noted to induce a mean of 0.44 log units (maximum 0.6 log units) of a defect in the contralateral eye. However, the contralateral RAPD disappears after the extraction of the cataract.[15]
RAPD in Eye Patching and Dark Adaptation[16]
A ptotic eyelid or eye patch increases retinal sensitivity due to dark adaptation and causes a false RAPD of up to 1.5 log units in the uncovered eye. The contralateral physiological afferent pupil must be allowed to resolve prior to commenting on the pupil. In most of the cases, this induced RAPD disappears within 30 minutes of examination in an illuminated room or removal of the eye patch.
Miscellaneous
RAPD in Amblyopia[17]
According to classic teaching, in amblyopia, there is no structural abnormality of the retina or the optic nerve, and hence RAPD should not be found. However, a study on amblyopic eyes showed that 'easily visible' RAPD (>0.3 log units) was noted in 29 of 55 subjects (52.7%), and a total of 45 of 55 patients (81.8%) had RAPD. Most of the cases had RAPD of a maximum of 0.6 log units. Some cases had minimal RAPD of <0.3 log units, which was detected by a modification of the standard RAPD test. There is no definite proven hypothesis on the cause of RAPD in amblyopic eyes. Various presumed hypothesis includes impairment of the function of the central retina[30] and impaired development of X ganglion cells in the fovea of the amblyopic eye.[31] There is no correlation between the severity of amblyopia and the grade of RAPD.
RAPD in Anisocoria[18][32]
In anisocoria, the retina in one eye is shaded by iris more than the other. This difference in retinal illumination can produce an RAPD in the eye with a smaller pupil. For every 1 mm difference in the size of pupils, a defect of 0.1 log units of RAPD is noted. Hence a difference of 3 mm between the pupils produces a clinically detectable RAPD. This artefactitious pupillary response can be alleviated by placing neutral density filters over the larger pupil at a rate of 1/10 of the log unit for every millimeter of anisocoria.
History and Physical
Patients with RAPD can present with normal visual function[8][33] or may have symptoms of an underlying disorder. RAPD is known to persist even when excellent recovery of vision has occurred after the resolution of an acute episode of optic neuritis.[34] RAPD alone does not cause anisocoria.
Ocular Examination
- Visual acuity: may range from perception of light (PL) to 20/20
- Ocular alignment: patients with long-standing amblyopia may manifest sensory exodeviation.
- Assessment of pupils:
- A weaker initial constriction with greater redilatation
- An initial stall with greater redilatation
- An initial immediate dilatation, known as pupillary escape
- Pupillary escape is an evident and specific sign of RAPD.
- Anterior segment examination: the presence of iris neovascularization should raise suspicion of ischemic CRVO
- Fundus evaluation: helps to identify underlying causes such as optic neuropathy (optic neuritis, ischemic optic neuropathy), retinal detachment, retinal vascular occlusion, glaucoma, and others.
Supportive Tests: Color vision, contrast vision, Visual evoked potential, Visual fields evaluation, MRI in cases of optic neuritis
Evaluation
A relative afferent pupillary defect usually indicates disease in the pre chiasmal visual pathway.[3] The ability to detect and quantify RAPD is an essential part of the neuro-ophthalmic examination.
Tests to Identify and Quantify RAPD
- Gunn test
- Kestenbaum-Gunn test / modified Gunn test
- Swinging flashlight test
- Pupillography
Use on neutral density filters and cross-polarized filters aid in the quantification of RAPD in conjunction with swinging flashlight test or Modified Gunn test.[32][35][32]
Modified Gunn Test / Kestenbaum Gunn Test
In this test, the pupillary reaction to prolonged light exposure is assessed. When any patient has unilateral optic nerve dysfunction or asymmetric involvement of optic neuropathy, with the patient in the light, covering the eyes alternatively reveals, that pupil of the normal eye constricts when it is uncovered, and the abnormal eye is covered. In contrast, the pupil of the eye with optic neuropathy dilates when it is uncovered, and the pupil of the normal eye is covered. Here the size of pupils is measured.
Swinging Flashlight Test[36][29]
Levatin modified the Gunn test for the detection of the relative afferent pupillary defect. He replaced alternate covering of eyes with alternate flashlight stimulation. The swinging flashlight test is superior to the Marcus Gunn test for the detection of RAPD.[37]
Procedure
- In a semi-dark room, ask the patient to fix at a letter or pattern on the distance chart (looking at a near object causes accommodative miosis)
- Shine a penlight or indirect ophthalmoscope light into one eye from below the patient's eye at a distance of 5 to 10 cm.
- After a pause of 3 seconds, quickly shift the light to the other eye.[38] Move the whole light from side to side. Do not swing the beam from one central axis, as this can stimulate the near response.
- Repeatedly alternate the light between the two eyes, with a pause of 3 seconds on each eye.
- Look for the change in pupil size as light is alternated.
An eye with RAPD will dilate as the consensual dilatation response due to light moving off the good eye overpowers the poor constriction (direct pupil light reflex) response from the affected eye.
Clinical grading of RAPD based on swinging flashlight test.[38] The grades are determined by averaging pupillary responses over a minimum of six swings of the light.
- Grade I: A weak initial constriction and greater redilatation
- Grade II: An initial stall and greater redilatation
- Grade III: An immediate dilatation
- Grade IV: An immediate pupillary dilatation (followed by secondary constriction) following prolonged illumination of a good eye for 6 seconds (bleaching of the normal eye)
- Grade V: An immediate pupillary dilatation with no secondary constriction following prolonged illumination of a good eye for 6 seconds
Grade IV and V are elicited after bleaching the photoreceptors of the good eye to reduce its pupil power.
Special Situations
- Detection of RAPD in a fixed pupil [29] – when one pupil cannot react due to posterior synechiae, trauma, or pharmacological miosis, we can still look for RAPD. In these situations, one should focus on the reactive pupil.
For example –
- If the afferent defect is present in the eye with a fixed pupil, when light is thrown onto the normal eye, its pupil constricts. When the light is moved to the abnormal eye, the pupil in the normal eye dilates or constricts less. Returning the light to the normal eye causes its pupil to constrict again.
- If the normal eye has fixed pupil, here, shifting the light from this eye to the other, the reactive pupil will dilate.
- In cases with subtle RAPD[17][29] – To unmask subtle RAPD, a Tilt test is performed.[39]
A neutral density filter of 0.3 log units is placed in front of the eye with suspected optic neuropathy. Then the swinging flashlight test is performed. Placing the neutral density filter in front of the weak eye further decreases the pupillomotor input of that eye and heightens the inter eye pupillomotor input difference. Thus RAPD is unmasked.
Quantification of RAPD Using Neutral Density Filters[32][38]
This is done by placing neutral density filters over the better eye and performing swinging flashlight test.
In this test, we dim the stimulus in the better eye by known amounts with neutral density filters (usually 0.3, 0.6, 0.9, and 1.2 log units) and perform the swinging flashlight test until the two eyes are balanced and no RAPD is noted. The density of the filter is recorded in log units as a measure of the size of the defect. The smallest defect that can be measured with confidence is 0.3 log units. It is important to avoid light spill at the edge of the filters as the light is swung from one eye to the other. It is difficult to visualize pupil beyond 1.2 log units of neutral density filter, necessitating the examiner to peek around the filter to watch the pupillary reflex. During the first attempt, it is better to overshoot the endpoint and induce an RAPD in the filtered eye; then, as one approaches the endpoint for the second time, the readings can be made more accurately.
Kestenbaum’s pupil number[24]
In patients with unilateral optic nerve disease, with one eye covered and other eye exposed to bright light, the pupil of affected eye settles at a larger diameter. This difference (in millimeters) in size of two pupils, is a measure of the difference in pupillomotor input between the two eyes. It roughly corresponds to the relative afferent pupillary defect measured in log units of neutral density filter.
Limitations
Swinging flashlight test is subject to various inconsistencies due to interindividual variability, the experience of clinical performing the test, ambient light conditions, and cooperation of the patient and lack of precise criteria for quantification. It is difficult to measure Kestenbaum's number or quantify relative afferent pupillary defect in children due to hippus and in adults due to miosis and poor iris mobility.[24]
Pupillography[40]
Modern pupillography uses a computerized device to simulate the swinging flashlight test and allows more accurate detection and quantification of RAPDs.[41] These are binocular pupillometers which provide information on pupil diameter, pupil diameter vs. time curves, asymmetry in pupillary responses. The data studied in pupillography includes average pupillary constriction amplitude (CA), constriction velocity (CV), and constriction onset latency (COL). CA is the best parameter for detecting RAPDs and differentiating patients from normal subjects.[42]
Treatment / Management
The treatment is directed towards the underlying cause.
Differential Diagnosis
Hippus
Random fluctuations of the pupil that are often observed under continual retinal illumination.[43]
Alternating Contraction Anisocoria
Direct pupillary constriction of the eye exceeds the consensual constriction of the contralateral pupil. This phenomenon may be unilateral or bilateral. In one subset of unilateral contraction anisocoria, the pupillary response of one side always exceeds that of the other eye. This subset is often confused with a relative pupillary afferent defect. The cause is hypothesized to be a lesion at the posterior commissure and one or both brachia of the superior colliculus due to damage of combination of both first and second-order neurons.[23]
Few diagnostic pearls:
- RAPD with normal visual acuity, color vision, field defects suspect higher-order lesion at the level of the pretectum
- RAPD in one eye and contralateral field defects or specifically temporal field defects - optic tract lesion
- RAPD and NVI (new vessels of the iris) - look for ischemic CRVO
- RAPD and anisocoria - Repeat test after 30 minutes of light adaptation
- Cataract and same eye RAPD - suspect posterior segment pathology
- Cataract and contralateral eye RAPD - can be a transient phenomenon or posterior segment lesion of the other eye such as recovered optic neuritis
Prognosis
In optic neuropathy, RAPD magnitude often correlates with the degree of visual acuity loss, while in maculopathy, vision is impaired to a greater magnitude compared to RAPD.[12]
In glaucoma – the presence of RAPD is objective evidence of visual field loss.[20] It is indicative of the earliest onset of optic nerve damage in patients with optic nerve hypertension.[44]
In retinal vein occlusion – An RAPD >0.9 log units is associated with extensive capillary nonperfusion and poor visual acuity (Usually< 3/60).[11] A significant increase in RAPD in a patient with non-ischemic CRVO is an early indicator of conversion to ischemic CRVO.
In retinal detachment – An RAPD usually occurs in retinal detachment involving the macula, and post-operative visual acuity is usually lower in detachments involving the macula.[45]
In cataract – if the patient has RAPD in the same eye as cataract, then post-operative visual gain tends to be poor due to associated defects in the anterior visual pathway.[15] A poor visual prognosis should always be explained if the eye with cataract shows RAPD.
Complications
The failure to detect this clinical sign results in complications secondary to the underlying etiology.
Deterrence and Patient Education
Measurement of RAPD has proven value in the prediction of visual field loss in certain optic nerve and retinal diseases.[5] RAPD is a very sensitive and specific test as a predictor of ischemia and in the differentiation of ischemic and nonischemic central retinal vein occlusion.[11] Thus, this simple test for RAPD detection can be used to counsel the patients.
Enhancing Healthcare Team Outcomes
One should not be in a hurry to dilate the eye, as one might miss important pupillary signs. In a patient admitted in the general ward or intensive care unit, the examination of pupillary reflexes by nurses and interns helps to identify certain neuro-ophthalmic conditions and enable prompt referral to the ophthalmologist. Certain tumors such as pituitary adenoma may remain silent and may be diagnosed on a detailed evaluation of a case with a relative afferent pupillary defect.