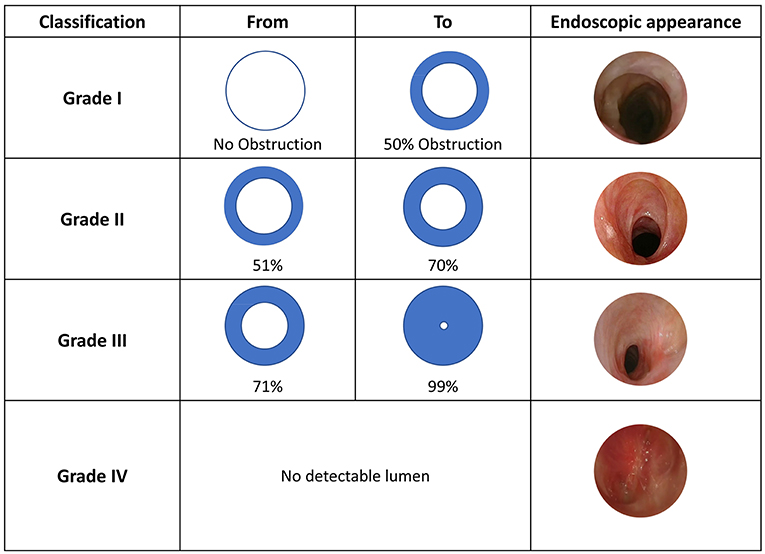
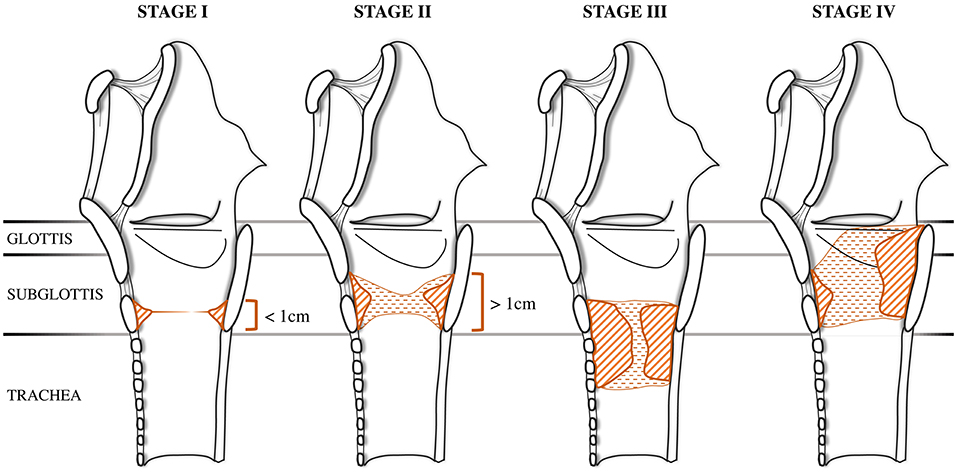
[1]
Courey MS. Airway obstruction. The problem and its causes. Otolaryngologic clinics of North America. 1995 Aug:28(4):673-84
[PubMed PMID: 7478630]
[2]
McCullagh KL, Shah RN, Huang BY. Anatomy of the Larynx and Cervical Trachea. Neuroimaging clinics of North America. 2022 Nov:32(4):809-829. doi: 10.1016/j.nic.2022.07.011. Epub
[PubMed PMID: 36244725]
[3]
Bacchin D, Aprile V, Lenzini A, Korasidis S, Mastromarino MG, Picchi A, Fanucchi O, Ribechini A, Ambrogi MC, Lucchi M. Surgical treatment of tracheal stenosis during Covid-19 era: a single-center experience and lessons learnt on the field. Updates in surgery. 2023 Sep:75(6):1681-1690. doi: 10.1007/s13304-023-01577-6. Epub 2023 Jul 17
[PubMed PMID: 37458903]
[4]
Pandya A, Sreevidya SR, Chaudhari N, Prajapati BJ, Gupta N. Laryngotracheal Stenosis: Our Experience in a Tertiary Care Hospital. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2023 Mar:75(1):39-44. doi: 10.1007/s12070-022-03445-y. Epub 2022 Dec 31
[PubMed PMID: 37007876]
[5]
Talatala ERR, Clark E, Ye W, Davis RJ, Hillel AT, Collins SL, Ramirez-Solano M, Sheng Q, Gelbard A. Localizing Hormone Receptor Expression to Cellular Compartments in Idiopathic Subglottic Stenosis. The Laryngoscope. 2023 Dec:133(12):3506-3511. doi: 10.1002/lary.30856. Epub 2023 Jun 29
[PubMed PMID: 37382162]
[6]
Krishnan A, Guenthart BA, Choi A, Trope W, Berry GJ, Pinezich MR, Vunjak-Novakovic G, Shaller B, Sung CK, Liou DZ, Damrose EJ, Lui NS. Tracheal stenosis and airway complications in the Coronavirus Disease-19 era. Annals of thoracic surgery short reports. 2023 Jun 7:():. doi: 10.1016/j.atssr.2023.05.013. Epub 2023 Jun 7
[PubMed PMID: 37360840]
[7]
Thawley SE, Ogura JH. Panel discussion: the management of advanced laryngotracheal stenosis. Use of the hyoid graft for treatment of laryngotracheal stenosis. The Laryngoscope. 1981 Feb:91(2):226-32
[PubMed PMID: 7464385]
[8]
Simpson GT, Strong MS, Healy GB, Shapshay SM, Vaughan CW. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. The Annals of otology, rhinology, and laryngology. 1982 Jul-Aug:91(4 Pt 1):384-8
[PubMed PMID: 7114718]
[9]
Prasanna Kumar S, Ravikumar A, Senthil K, Somu L, Nazrin MI. Role of Montgomery T-tube stent for laryngotracheal stenosis. Auris, nasus, larynx. 2014 Apr:41(2):195-200. doi: 10.1016/j.anl.2013.10.008. Epub 2013 Oct 27
[PubMed PMID: 24172854]
[10]
Tinga S, Thieman Mankin KM, Peycke LE, Cohen ND. Comparison of Outcome After Use of Extra-Luminal Rings and Intra-Luminal Stents for Treatment of Tracheal Collapse in Dogs. Veterinary surgery : VS. 2015 Oct:44(7):858-65. doi: 10.1111/vsu.12365. Epub 2015 Aug 6
[PubMed PMID: 26249528]
[11]
Folch E, Keyes C. Airway stents. Annals of cardiothoracic surgery. 2018 Mar:7(2):273-283. doi: 10.21037/acs.2018.03.08. Epub
[PubMed PMID: 29707506]
[12]
Sung YN, Kim Y, Cho KJ, Sim J. Assessment of thyroid cartilage ossification pattern in cancer patients: A suggestion of active ossification by tumor progression. Head & neck. 2023 Aug:45(8):2040-2046. doi: 10.1002/hed.27430. Epub 2023 Jun 23
[PubMed PMID: 37353470]
[13]
Paniello RC, Brookes S, Zhang H, Halum S. Enhanced Abductor Function in Bilateral Vocal Fold Paralysis with Muscle Stem Cells. The Laryngoscope. 2024 Jan:134(1):324-328. doi: 10.1002/lary.30885. Epub 2023 Jul 18
[PubMed PMID: 37462328]
[14]
Coviello CM, Sheehan C, Hernandez DJ, Liou NE, Sandulache VC, Haskins AD, Sturgis EM, Huang AT. Outcome Comparison of Functional Laryngectomy for the Dysfunctional Larynx to Salvage Laryngectomy. The Laryngoscope. 2024 Jan:134(1):222-227. doi: 10.1002/lary.30844. Epub 2023 Jun 22
[PubMed PMID: 37345670]
[15]
Suzuki K, Yambe N, Hojo K, Komatsu Y, Serikawa M, Usami A. Anatomical morphometry for Cricothyrotomy puncture and incision. BMC surgery. 2023 Jul 12:23(1):198. doi: 10.1186/s12893-023-02100-9. Epub 2023 Jul 12
[PubMed PMID: 37438728]
[16]
Voets PJ, van Helvoort HA. The role of equal pressure points in understanding pulmonary diseases. Advances in physiology education. 2013 Sep:37(3):266-7. doi: 10.1152/advan.00014.2013. Epub
[PubMed PMID: 24022774]
Level 3 (low-level) evidence
[17]
Myer CM 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. The Annals of otology, rhinology, and laryngology. 1994 Apr:103(4 Pt 1):319-23
[PubMed PMID: 8154776]
[18]
Li Y, Zhu M, Chen J, Ren K, Wan L, Lu H, Ren J, Han X. Single Y-shaped tracheal self-expandable metallic stent for emergent carinal stenosis combined with stenosis of the right main and intermediate bronchi. Medicine. 2020 May 29:99(22):e20498. doi: 10.1097/MD.0000000000020498. Epub
[PubMed PMID: 32481466]
[19]
Tsakiridis K, Darwiche K, Visouli AN, Zarogoulidis P, Machairiotis N, Christofis C, Stylianaki A, Katsikogiannis N, Mpakas A, Courcoutsakis N, Zarogoulidis K. Management of complex benign post-tracheostomy tracheal stenosis with bronchoscopic insertion of silicon tracheal stents, in patients with failed or contraindicated surgical reconstruction of trachea. Journal of thoracic disease. 2012 Nov:4 Suppl 1(Suppl 1):32-40. doi: 10.3978/j.issn.2072-1439.2012.s002. Epub
[PubMed PMID: 23304439]
[20]
Thomas GK, Stevens MH. Stenting in experimental laryngeal injuries. Archives of otolaryngology (Chicago, Ill. : 1960). 1975 Apr:101(4):217-21
[PubMed PMID: 1120009]
[21]
Froehlich P, Truy E, Stamm D, Floret D, Morgon A. Role of long-term stenting in treatment of pediatric subglottic stenosis. International journal of pediatric otorhinolaryngology. 1993 Oct:27(3):273-80
[PubMed PMID: 8270365]
[22]
Wahidi MM, Ernst A. The Montgomery T-tube tracheal stent. Clinics in chest medicine. 2003 Sep:24(3):437-43
[PubMed PMID: 14535218]
[23]
Hu H,Zhang J,Wu F,Chen E, Application of the Montgomery T-tube in subglottic tracheal benign stenosis. Journal of thoracic disease. 2018 May;
[PubMed PMID: 29997975]
[24]
Zalzal GH, Grundfast KM. Broken Aboulker stents in the tracheal lumen. International journal of pediatric otorhinolaryngology. 1988 Nov:16(2):125-30
[PubMed PMID: 3209360]
[25]
Wilcox JD, Nassar M. A Modified Laryngeal Stent for Glotto-Subglottic Stenosis: A Novel Stent for Better Outcomes. Ear, nose, & throat journal. 2021 Sep:100(5_suppl):399S-403S. doi: 10.1177/0145561319883074. Epub 2019 Oct 22
[PubMed PMID: 31637951]
[26]
Smith DF, de Alarcon A, Jefferson ND, Tabangin ME, Rutter MJ, Cotton RT, Hart CK. Short- versus Long-term Stenting in Children with Subglottic Stenosis Undergoing Laryngotracheal Reconstruction. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2018 Feb:158(2):375-380. doi: 10.1177/0194599817737757. Epub 2017 Oct 24
[PubMed PMID: 29064319]
[27]
Monnier P. A new stent for the management of adult and pediatric laryngotracheal stenosis. The Laryngoscope. 2003 Aug:113(8):1418-22
[PubMed PMID: 12897569]
[28]
Alshammari J, Monnier P. Airway stenting with the LT-Mold™ for severe glotto-subglottic stenosis or intractable aspiration: experience in 65 cases. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2012 Dec:269(12):2531-8. doi: 10.1007/s00405-012-2080-x. Epub 2012 Jun 22
[PubMed PMID: 22722945]
Level 3 (low-level) evidence
[29]
Zalzal GH. Stenting for pediatric laryngotracheal stenosis. The Annals of otology, rhinology, and laryngology. 1992 Aug:101(8):651-5
[PubMed PMID: 1497269]
[30]
Sweed AH, Mobashir M, Mohamed AES, Elsayed AI, Elmalt A, Elshora ME. Simple Endoscopic Application of Laryngeal Keel Stent. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2022 Feb:166(2):399-402. doi: 10.1177/01945998211002162. Epub 2021 Mar 23
[PubMed PMID: 33752537]
[31]
Dalar L, Tural Önür S, Özdemir C, Sökücü SN, Karasulu AL, Altin S. Is silicone stent insertion a clinically useful option for tracheobronchomalacia? Turkish journal of medical sciences. 2016 Feb 17:46(2):437-42. doi: 10.3906/sag-1412-104. Epub 2016 Feb 17
[PubMed PMID: 27511508]
[32]
Miwa K, Takamori S, Hayashi A, Fukunaga M, Shirouzu K. Fixation of silicone stents in the subglottic trachea: preventing stent migration using a fixation apparatus. The Annals of thoracic surgery. 2004 Dec:78(6):2188-90
[PubMed PMID: 15561074]
[33]
Andreetti C, Menna C, D'Andrilli A, Ibrahim M, Venuta F, Santini M, Rendina EA, Fiorelli A. A modified technique to simplify external fixation of the subglottic silicone stent. Interactive cardiovascular and thoracic surgery. 2018 Dec 1:27(6):878-880. doi: 10.1093/icvts/ivy178. Epub
[PubMed PMID: 29868866]
[34]
McGinniss JE, Imai I, Simon-Soro A, Brown MC, Knecht VR, Frye L, Ravindran PM, Dothard MI, Wadell DA, Sohn MB, Li H, Christie JD, Diamond JM, Haas AR, Lanfranco AR, DiBardino DM, Bushman FD, Collman RG. Molecular analysis of the endobronchial stent microbial biofilm reveals bacterial communities that associate with stent material and frequent fungal constituents. PloS one. 2019:14(5):e0217306. doi: 10.1371/journal.pone.0217306. Epub 2019 May 29
[PubMed PMID: 31141557]
[35]
Cotton RT, Myer CM 3rd, Bratcher GO, Fitton CM. Anterior cricoid split, 1977-1987. Evolution of a technique. Archives of otolaryngology--head & neck surgery. 1988 Nov:114(11):1300-2
[PubMed PMID: 3166763]
[36]
Kurien M, Raviraj R, Mathew J, Kaliaperumal I, Ninan S. Modified endotracheal tube: emergency alternative to paediatric tracheostomy tube. The Journal of laryngology and otology. 2011 Jul:125(7):729-31. doi: 10.1017/S0022215111000636. Epub 2011 Apr 13
[PubMed PMID: 21486520]
[37]
Awasthy N, Arora HS, Radhakrishnan S. Endotracheal tube as tracheal stent in vascular ring. Asian cardiovascular & thoracic annals. 2016 Feb:24(2):195-7. doi: 10.1177/0218492314554238. Epub 2014 Oct 3
[PubMed PMID: 25281764]
[38]
Phillips MJ. Stenting therapy for stenosing airway diseases. Respirology (Carlton, Vic.). 1998 Dec:3(4):215-9
[PubMed PMID: 10201046]
[39]
Lim LH, Cotton RT, Azizkhan RG, Wood RE, Cohen AP, Rutter MJ. Complications of metallic stents in the pediatric airway. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2004 Oct:131(4):355-61
[PubMed PMID: 15467599]
[40]
Chin CS, Litle V, Yun J, Weiser T, Swanson SJ. Airway stents. The Annals of thoracic surgery. 2008 Feb:85(2):S792-6. doi: 10.1016/j.athoracsur.2007.11.051. Epub
[PubMed PMID: 18222219]
[41]
Hramiec JE, Haasler GB. Tracheal wire stent complications in malacia: implications of position and design. The Annals of thoracic surgery. 1997 Jan:63(1):209-12; discussion 213
[PubMed PMID: 8993267]
[42]
Bi Y, Li J, Bai L, Han X, Ren J. Long-term outcomes of tracheal stents removal under fluoroscopy guidance: comparison of tracheal fistulas and tracheal stenosis. BMC pulmonary medicine. 2021 Jan 7:21(1):14. doi: 10.1186/s12890-020-01349-7. Epub 2021 Jan 7
[PubMed PMID: 33413278]
[43]
Choi MJ, Kang H. CT Findings of Central Airway Lesions Causing Airway Stenosis-Visualization and Quantification: A Pictorial Essay. Taehan Yongsang Uihakhoe chi. 2021 Nov:82(6):1441-1476. doi: 10.3348/jksr.2020.0212. Epub 2021 Sep 16
[PubMed PMID: 36238875]
[44]
Nair S, Mohan S, Mandal G, Nilakantan A. Tracheal stenosis: our experience at a tertiary care centre in India with special regard to cause and management. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2014 Jan:66(1):51-6. doi: 10.1007/s12070-013-0663-5. Epub 2013 Jun 15
[PubMed PMID: 24605302]
[45]
Moore AE, Walker A, Kanotra SP. Endoscopic Versus Open Surgical Intervention for Congenital Laryngeal Webs: A Systematic Review and Meta-Analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2023 Aug:169(2):227-233. doi: 10.1002/ohn.279. Epub 2023 Feb 7
[PubMed PMID: 36939597]
Level 1 (high-level) evidence
[46]
Lorenz RR. The Evolution and Outcomes of the "Maddern Procedure" for the Treatment of Subglottic Stenosis. The Laryngoscope. 2023 Nov:133(11):3100-3108. doi: 10.1002/lary.30752. Epub 2023 May 17
[PubMed PMID: 37194674]
[47]
Rutter MJ, Cotton RT. The use of posterior cricoid grafting in managing isolated posterior glottic stenosis in children. Archives of otolaryngology--head & neck surgery. 2004 Jun:130(6):737-9
[PubMed PMID: 15210555]
[48]
Wei W, Li X, Feng L, Jiao J, Li W, Cai Y, Fang R, Han Y. The effect of intraoperative transnasal humidified rapid-insufflation ventilatory exchange on emergence from general anesthesia in patients undergoing microlaryngeal surgery: a randomized controlled trial. BMC anesthesiology. 2023 Jun 13:23(1):202. doi: 10.1186/s12871-023-02169-y. Epub 2023 Jun 13
[PubMed PMID: 37312020]
Level 1 (high-level) evidence
[49]
Davis RJ, Lina I, Motz K, Gelbard A, Lorenz RR, Sandhu GS, Hillel AT. Endoscopic Resection and Mucosal Reconstitution With Epidermal Grafting: A Pilot Study in Idiopathic Subglottic Stenosis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2022 May:166(5):917-926. doi: 10.1177/01945998211028163. Epub 2021 Jul 13
[PubMed PMID: 34253069]
Level 3 (low-level) evidence
[50]
Liang KY, Nelson RC, Bryson PC, Lorenz RR. High Tracheal Resection With Intralaryngeal Extension as an Alternative to Cricotracheal Resection for Treatment of Subglottic Stenosis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2023 May:168(5):1139-1145. doi: 10.1002/ohn.180. Epub 2023 Jan 19
[PubMed PMID: 36939535]
[51]
Redmann AJ, Moore C, Kou YF, Tabangin ME, Wilcox L, Smith MM, Hart CK, Rutter MJ, de Alarcon A. Revision Endoscopic Posterior Costal Cartilage Grafting: Is It Feasible? Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2023 Aug:169(2):432-434. doi: 10.1002/ohn.275. Epub 2023 Feb 7
[PubMed PMID: 36939543]
[52]
Jethwa AR, Hasan W, Palme CE, Mäkitie AA, Espin-Garcia O, Goldstein DP, Gilbert RW, Keshavjee S, Pierre A, Gullane PJ. Cricotracheal resection for adult subglottic stenosis: Factors predicting treatment failure. The Laryngoscope. 2020 Jul:130(7):1634-1639. doi: 10.1002/lary.28273. Epub 2019 Sep 9
[PubMed PMID: 31498456]
[53]
Tan LT, Xie Y, Li Q, Chen C. [Outcomes of laryngotracheal reconstruction with anterior and posterior costal cartilage grafts in severe pediatric subglottic stenosis or laryngeal web]. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2023 Jul 7:58(7):699-704. doi: 10.3760/cma.j.cn115330-20221124-00708. Epub
[PubMed PMID: 37455115]
[54]
Ravikumar N, Ho E, Wagh A, Murgu S. The role of bronchoscopy in the multidisciplinary approach to benign tracheal stenosis. Journal of thoracic disease. 2023 Jul 31:15(7):3998-4015. doi: 10.21037/jtd-22-1734. Epub 2023 Jun 28
[PubMed PMID: 37559626]
[55]
Hautmann H, Bauer M, Pfeifer KJ, Huber RM. Flexible bronchoscopy: a safe method for metal stent implantation in bronchial disease. The Annals of thoracic surgery. 2000 Feb:69(2):398-401
[PubMed PMID: 10735670]
[56]
Li J, Xu K, Li Z, Li Y, Zhou X, Liu J, Yao Y, Liu Z, Jiao DC, Han X. Intraoperative cone beam computed tomography of tracheal stenting for stenosis and fistula diseases: a retrospective cohort study. Quantitative imaging in medicine and surgery. 2022 May:12(5):2709-2720. doi: 10.21037/qims-21-858. Epub
[PubMed PMID: 35502374]
Level 2 (mid-level) evidence
[57]
Marchese R, Poidomani G, Palumbo VD, Lo Nigro C, Caterino U, Lo Monte AI, Cajozzo M. Secondary Carina and Lobar Bronchi Stenting in Patients with Advanced Lung Cancer: Is It Worth the Effort? A Clinical Experience. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2020 Dec 20:26(6):320-326. doi: 10.5761/atcs.oa.19-00040. Epub 2020 May 18
[PubMed PMID: 32418926]
[58]
Jung HS, Chae G, Kim JH, Park CY, Lim S, Park SE, Kim HC, Lee YJ, Kang SK, Kim DH, Lee Y, Lee T. The mechanical characteristics and performance evaluation of a newly developed silicone airway stent (GINA stent). Scientific reports. 2021 Apr 12:11(1):7958. doi: 10.1038/s41598-021-87142-w. Epub 2021 Apr 12
[PubMed PMID: 33846477]
[59]
Lin J, Frye L. The intersection of bronchoscopy and extracorporeal membrane oxygenation. Journal of thoracic disease. 2021 Aug:13(8):5176-5182. doi: 10.21037/jtd-2019-ipicu-08. Epub
[PubMed PMID: 34527357]
[60]
Ren J, Xu Y, Zhiyi G, Ren T, Ren J, Wang K, Luo Y, Zhu M, Tan Q. Reconstruction of the trachea and carina: Surgical reconstruction, autologous tissue transplantation, allograft transplantation, and bioengineering. Thoracic cancer. 2022 Feb:13(3):284-295. doi: 10.1111/1759-7714.14315. Epub 2022 Jan 13
[PubMed PMID: 35023311]
[61]
Khalid T, Soriano L, Lemoine M, Cryan SA, O'Brien FJ, O'Leary C. Development of tissue-engineered tracheal scaffold with refined mechanical properties and vascularisation for tracheal regeneration. Frontiers in bioengineering and biotechnology. 2023:11():1187500. doi: 10.3389/fbioe.2023.1187500. Epub 2023 Jun 6
[PubMed PMID: 37346796]
[62]
Murray P. The trachea transplant scandal and "compassionate use". BMJ (Clinical research ed.). 2023 Aug 10:382():1808. doi: 10.1136/bmj.p1808. Epub 2023 Aug 10
[PubMed PMID: 37562813]
[63]
Li ZM, Jiao DC, Han XW, Lu HB, Ren KW, Yang H. Clinical evaluation the success rate and complications of fluoroscopically guided removal of tracheal tube metallic stents. Journal of cardiothoracic surgery. 2021 Mar 25:16(1):54. doi: 10.1186/s13019-021-01444-8. Epub 2021 Mar 25
[PubMed PMID: 33766043]
[64]
Nouraei SA, Petrou MA, Randhawa PS, Singh A, Howard DJ, Sandhu GS. Bacterial colonization of airway stents: a promoter of granulation tissue formation following laryngotracheal reconstruction. Archives of otolaryngology--head & neck surgery. 2006 Oct:132(10):1086-90
[PubMed PMID: 17043256]
[65]
Rampey AM, Silvestri GA, Gillespie MB. Combined endoscopic and open approach to the removal of expandable metallic tracheal stents. Archives of otolaryngology--head & neck surgery. 2007 Jan:133(1):37-41
[PubMed PMID: 17224520]
[66]
Pasick LJ, Anis MM, Rosow DE. An Updated Review of Subglottic Stenosis: Etiology, Evaluation, and Management. Current pulmonology reports. 2022:11(2):29-38. doi: 10.1007/s13665-022-00286-6. Epub 2022 Mar 3
[PubMed PMID: 35261874]
[67]
Ho S, Goh SK, Ng AW, Tai DY, Lim AY, Kor AC, Sien Zin NN, Abisheganaden J, Verma A. Long-term tolerance of a fractured self-expanding metal stent in a patient with adenoid cystic carcinoma. Respiratory medicine case reports. 2019:28():100960. doi: 10.1016/j.rmcr.2019.100960. Epub 2019 Oct 25
[PubMed PMID: 31720207]
Level 3 (low-level) evidence
[68]
Sriram K, Robinson P. Recurrent airway obstructions in a patient with benign tracheal stenosis and a silicone airway stent: a case report. Cases journal. 2008 Oct 7:1(1):226. doi: 10.1186/1757-1626-1-226. Epub 2008 Oct 7
[PubMed PMID: 18840299]
Level 3 (low-level) evidence
[69]
Biswas A, Jantz MA, Fernandez-Bussy S, Flanagan M, Mehta HJ. Repositioning of migrated self-expanding metallic tracheobronchial stent: predictors of a successful maneuver and its impact on survival. Journal of thoracic disease. 2020 May:12(5):1866-1876. doi: 10.21037/jtd-20-608. Epub
[PubMed PMID: 32642090]
[70]
Grant CA, Dempsey G, Harrison J, Jones T. Tracheo-innominate artery fistula after percutaneous tracheostomy: three case reports and a clinical review. British journal of anaesthesia. 2006 Jan:96(1):127-31
[PubMed PMID: 16299043]
Level 3 (low-level) evidence
[71]
Chaddha U, Hogarth DK, Murgu S. Perspective on airway stenting in inoperable patients with tracheoesophageal fistula after curative-intent treatment for esophageal cancer. Journal of thoracic disease. 2019 May:11(5):2165-2174. doi: 10.21037/jtd.2018.12.128. Epub
[PubMed PMID: 31285911]
Level 3 (low-level) evidence