[1]
Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant Cell Tumor of Bone - An Overview. The archives of bone and joint surgery. 2016 Jan:4(1):2-9
[PubMed PMID: 26894211]
Level 3 (low-level) evidence
[2]
Ghostine B, Sebaaly A, Ghanem I. Multifocal metachronous giant cell tumor: case report and review of the literature. Case reports in medicine. 2014:2014():678035. doi: 10.1155/2014/678035. Epub 2014 Jan 5
[PubMed PMID: 24511316]
Level 3 (low-level) evidence
[3]
Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. International orthopaedics. 2006 Dec:30(6):484-9
[PubMed PMID: 17013643]
[4]
McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE, Dolecek TA, Kruchko C, Engelhard HH, Villano JL. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro-oncology. 2012 Sep:14(9):1194-200. doi: 10.1093/neuonc/nos155. Epub 2012 Aug 6
[PubMed PMID: 22869621]
[5]
Chan JY. A clinical overview of centrosome amplification in human cancers. International journal of biological sciences. 2011:7(8):1122-44
[PubMed PMID: 22043171]
Level 3 (low-level) evidence
[6]
Schwartz HS, Juliao SF, Sciadini MF, Miller LK, Butler MG. Telomerase activity and oncogenesis in giant cell tumor of bone. Cancer. 1995 Mar 1:75(5):1094-9
[PubMed PMID: 7850706]
[7]
Mavrogenis AF, Igoumenou VG, Megaloikonomos PD, Panagopoulos GN, Papagelopoulos PJ, Soucacos PN. Giant cell tumor of bone revisited. SICOT-J. 2017:3():54. doi: 10.1051/sicotj/2017041. Epub 2017 Sep 14
[PubMed PMID: 28905737]
[8]
Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. International orthopaedics. 2009 Apr:33(2):497-501. doi: 10.1007/s00264-008-0560-9. Epub 2008 May 7
[PubMed PMID: 18461324]
[9]
Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clinical orthopaedics and related research. 2010 Mar:468(3):827-33. doi: 10.1007/s11999-009-0966-8. Epub 2009 Jul 14
[PubMed PMID: 19597900]
[10]
Hasan O, Ali M, Mustafa M, Ali A, Umer M. Treatment and recurrence of giant cell tumors of bone - A retrospective cohort from a developing country. Annals of medicine and surgery (2012). 2019 Dec:48():29-34. doi: 10.1016/j.amsu.2019.10.010. Epub 2019 Oct 15
[PubMed PMID: 31687136]
Level 2 (mid-level) evidence
[11]
Patel MT, Nayak MR. Unusual Presentation of Giant Cell Tumor in Skeletally Immature Patient in Diaphysis of Ulna. Journal of orthopaedic case reports. 2015 Apr-Jun:5(2):28-31. doi: 10.13107/jocr.2250-0685.266. Epub
[PubMed PMID: 27299037]
Level 3 (low-level) evidence
[12]
Shekhar A, Murgod G, Korlhalli S. Synchronous Multicentric Giant Cell Tumour (GCT)-A Rare Case Report. Journal of clinical and diagnostic research : JCDR. 2014 Feb:8(2):185-6. doi: 10.7860/JCDR/2014/8153.4055. Epub 2014 Feb 3
[PubMed PMID: 24701530]
Level 3 (low-level) evidence
[13]
Verma V, Puri A, Shah S, Rekhi B, Gulia A. Giant Cell Tumor Developing in Paget's Disease of Bone: A Case Report with Review of Literature. Journal of orthopaedic case reports. 2016 Sep-Oct:6(4):103-107. doi: 10.13107/jocr.2250-0685.594. Epub
[PubMed PMID: 28164066]
Level 3 (low-level) evidence
[14]
Dufresne A, Derbel O, Cassier P, Vaz G, Decouvelaere AV, Blay JY. Giant-cell tumor of bone, anti-RANKL therapy. BoneKEy reports. 2012 Sep 5:1():149. doi: 10.1038/bonekey.2012.149. Epub 2012 Sep 5
[PubMed PMID: 24363925]
[15]
Morgan T, Atkins GJ, Trivett MK, Johnson SA, Kansara M, Schlicht SL, Slavin JL, Simmons P, Dickinson I, Powell G, Choong PF, Holloway AJ, Thomas DM. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. The American journal of pathology. 2005 Jul:167(1):117-28
[PubMed PMID: 15972958]
[16]
Mukaihara K, Suehara Y, Kohsaka S, Akaike K, Tanabe Y, Kubota D, Ishii M, Fujimura T, Kazuno S, Okubo T, Takagi T, Yao T, Kaneko K, Saito T. Protein Expression Profiling of Giant Cell Tumors of Bone Treated with Denosumab. PloS one. 2016:11(2):e0148401. doi: 10.1371/journal.pone.0148401. Epub 2016 Feb 10
[PubMed PMID: 26863138]
[17]
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Archives of biochemistry and biophysics. 2008 May 15:473(2):139-46. doi: 10.1016/j.abb.2008.03.018. Epub 2008 Mar 25
[PubMed PMID: 18395508]
[18]
Lindeman JH, Hanemaaijer R, Mulder A, Dijkstra PD, Szuhai K, Bromme D, Verheijen JH, Hogendoorn PC. Cathepsin K is the principal protease in giant cell tumor of bone. The American journal of pathology. 2004 Aug:165(2):593-600
[PubMed PMID: 15277232]
[19]
Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, Nik-Zainal S, Martin S, McLaren S, Goodie V, Robinson B, Butler A, Teague JW, Halai D, Khatri B, Myklebost O, Baumhoer D, Jundt G, Hamoudi R, Tirabosco R, Amary MF, Futreal PA, Stratton MR, Campbell PJ, Flanagan AM. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nature genetics. 2013 Dec:45(12):1479-82. doi: 10.1038/ng.2814. Epub 2013 Oct 27
[PubMed PMID: 24162739]
[20]
Steensma MR, Tyler WK, Shaber AG, Goldring SR, Ross FP, Williams BO, Healey JH, Purdue PE. Targeting the giant cell tumor stromal cell: functional characterization and a novel therapeutic strategy. PloS one. 2013:8(7):e69101. doi: 10.1371/journal.pone.0069101. Epub 2013 Jul 26
[PubMed PMID: 23922683]
[21]
Kushlinskii NE, Alferov AA, Timofeev YS, Gershtein ES, Bulycheva IV, Bondarev AV, Shchupak MY, Sokolov NY, Polikarpova SB, Efimova MM, Dzampaev AA, Sushentsov EA, Aliev MD, Musaev ER. Key Immune Checkpoint PD-1/PD-L1 Signaling Pathway Components in the Blood Serum from Patients with Bone Tumors. Bulletin of experimental biology and medicine. 2020 Nov:170(1):64-68. doi: 10.1007/s10517-020-05005-2. Epub 2020 Nov 24
[PubMed PMID: 33231796]
[22]
Metovic J, Annaratone L, Linari A, Osella-Abate S, Musuraca C, Veneziano F, Vignale C, Bertero L, Cassoni P, Ratto N, Comandone A, Grignani G, Piana R, Papotti M. Prognostic role of PD-L1 and immune-related gene expression profiles in giant cell tumors of bone. Cancer immunology, immunotherapy : CII. 2020 Sep:69(9):1905-1916. doi: 10.1007/s00262-020-02594-9. Epub 2020 May 6
[PubMed PMID: 32377818]
[23]
van der Heijden L, Lipplaa A, van Langevelde K, Bovée JVMG, van de Sande MAJ, Gelderblom H. Updated concepts in treatment of giant cell tumor of bone. Current opinion in oncology. 2022 Jul 1:34(4):371-378. doi: 10.1097/CCO.0000000000000852. Epub
[PubMed PMID: 35837707]
Level 3 (low-level) evidence
[24]
Kundu ZS, Sen R, Dhiman A, Sharma P, Siwach R, Rana P. Effect of Intravenous Zoledronic Acid on Histopathology and Recurrence after Extended Curettage in Giant Cell Tumors of Bone: A Comparative Prospective Study. Indian journal of orthopaedics. 2018 Jan-Feb:52(1):45-50. doi: 10.4103/ortho.IJOrtho_216_17. Epub
[PubMed PMID: 29416169]
Level 2 (mid-level) evidence
[25]
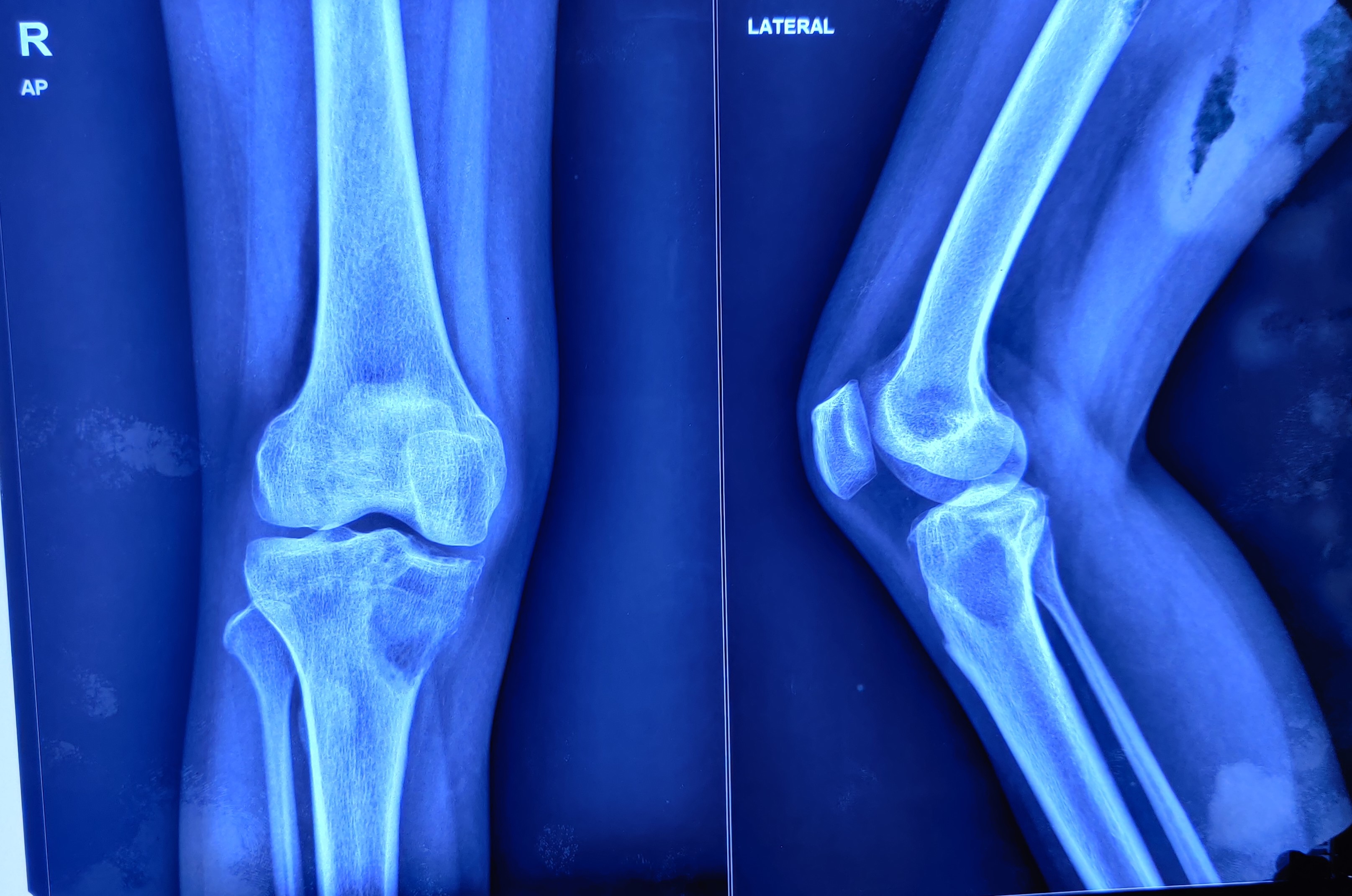
Purohit S, Pardiwala DN. Imaging of giant cell tumor of bone. Indian journal of orthopaedics. 2007 Apr:41(2):91-6. doi: 10.4103/0019-5413.32037. Epub
[PubMed PMID: 21139758]
[26]
Zhou Z, Li Y, Wang X, Hu J, Kuang M, Wang Z, Li S, Xu W, Xiao J. ALCAM(+) stromal cells: role in giant cell tumor of bone progression. Cell death & disease. 2018 Feb 20:9(3):299. doi: 10.1038/s41419-018-0361-z. Epub 2018 Feb 20
[PubMed PMID: 29463803]
[27]
Salerno M, Avnet S, Alberghini M, Giunti A, Baldini N. Histogenetic characterization of giant cell tumor of bone. Clinical orthopaedics and related research. 2008 Sep:466(9):2081-91. doi: 10.1007/s11999-008-0327-z. Epub 2008 Jun 10
[PubMed PMID: 18543051]
[28]
Yoshida H, Akeho M, Yumoto T. Giant cell tumor bone. Enzyme histochemical, biochemical and tissue culture studies. Virchows Archiv. A, Pathological anatomy and histology. 1982:395(3):319-30
[PubMed PMID: 6287714]
[30]
Schwartz HS, Dahir GA, Butler MG. Telomere reduction in giant cell tumor of bone and with aging. Cancer genetics and cytogenetics. 1993 Dec:71(2):132-8
[PubMed PMID: 8281516]
[31]
Wu PF, Tang JY, Li KH. RANK pathway in giant cell tumor of bone: pathogenesis and therapeutic aspects. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015 Feb:36(2):495-501. doi: 10.1007/s13277-015-3094-y. Epub 2015 Jan 25
[PubMed PMID: 25618600]
[32]
Wu R, Wu X, Weng X, Xiu Y, Xu G, Liu X, Liu X. Giant cell tumor of bone with H3F3B mutation: A case report. Medicine. 2023 Feb 17:102(7):e32995. doi: 10.1097/MD.0000000000032995. Epub
[PubMed PMID: 36800629]
Level 3 (low-level) evidence
[33]
Yakoub MA, Torrence D, Hwang S, Bartelstein M, Healey JH, Hameed M. Giant-cell-poor giant cell tumor of bone: report of two cases and literature review. Skeletal radiology. 2023 Sep:52(9):1791-1798. doi: 10.1007/s00256-023-04292-w. Epub 2023 Feb 14
[PubMed PMID: 36781420]
Level 3 (low-level) evidence
[34]
Larsson SE, Lorentzon R, Boquist L. Giant-cell tumor of bone. A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. The Journal of bone and joint surgery. American volume. 1975 Mar:57(2):167-73
[PubMed PMID: 1112843]
Level 3 (low-level) evidence
[35]
Jeys LM, Suneja R, Chami G, Grimer RJ, Carter SR, Tillman RM. Impending fractures in giant cell tumours of the distal femur: incidence and outcome. International orthopaedics. 2006 Apr:30(2):135-8
[PubMed PMID: 16474936]
[36]
Lewis VO, Wei A, Mendoza T, Primus F, Peabody T, Simon MA. Argon beam coagulation as an adjuvant for local control of giant cell tumor. Clinical orthopaedics and related research. 2007 Jan:454():192-7
[PubMed PMID: 16957652]
[37]
Hoeffel JC, Galloy MA, Grignon Y, Chastagner P, Floquet J, Mainard L, Kadiri R. Giant cell tumor of bone in children and adolescents. Revue du rhumatisme (English ed.). 1996 Oct:63(9):618-23
[PubMed PMID: 8938873]
[38]
Shih HN, Hsu RW, Sim FH. Excision curettage and allografting of giant cell tumor. World journal of surgery. 1998 May:22(5):432-7
[PubMed PMID: 9564283]
[39]
Bridge JA, Neff JR, Mouron BJ. Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. Cancer genetics and cytogenetics. 1992 Jan:58(1):2-13
[PubMed PMID: 1728946]
[40]
Osaka S, Toriyama S. Surgical treatment of giant cell tumors of the pelvis. Clinical orthopaedics and related research. 1987 Sep:(222):123-31
[PubMed PMID: 3621712]
[41]
Eckardt JJ, Grogan TJ. Giant cell tumor of bone. Clinical orthopaedics and related research. 1986 Mar:(204):45-58
[PubMed PMID: 3514036]
[42]
Tornberg DN, Dick HM, Johnston AD. Multicentric giant-cell tumors in the long bones. A case report. The Journal of bone and joint surgery. American volume. 1975 Apr:57(3):420-2
[PubMed PMID: 1123400]
Level 3 (low-level) evidence
[43]
Hayashida K, Kawabata Y, Yoshida T, Saito K, Fujita S, Choe H, Kato I, Takeyama M, Inaba Y. Characteristics of Patients with Giant Cell Tumor of Bone and High Serum Tartrate-Resistant Acid Phosphatase 5b Levels: Comparison of Tumor Volume and Clinical Factors. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2022:89(6):572-579. doi: 10.1272/jnms.JNMS.2022_89-611. Epub
[PubMed PMID: 36725001]
[44]
Pereira HM, Marchiori E, Severo A. Magnetic resonance imaging aspects of giant-cell tumours of bone. Journal of medical imaging and radiation oncology. 2014 Dec:58(6):674-8. doi: 10.1111/1754-9485.12249. Epub 2014 Sep 25
[PubMed PMID: 25256094]
[45]
Cavanna L, Biasini C, Monfredo M, Maniscalco P, Mori M. Giant cell tumor of bone. The oncologist. 2014 Nov:19(11):1207. doi: 10.1634/theoncologist.2014-0267. Epub
[PubMed PMID: 25378541]
[46]
Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV. Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. The American journal of surgical pathology. 2015 Nov:39(11):1576-83. doi: 10.1097/PAS.0000000000000512. Epub
[PubMed PMID: 26457357]
[47]
Fellenberg J, Sähr H, Mancarella D, Plass C, Lindroth AM, Westhauser F, Lehner B, Ewerbeck V. Knock-down of oncohistone H3F3A-G34W counteracts the neoplastic phenotype of giant cell tumor of bone derived stromal cells. Cancer letters. 2019 Apr 28:448():61-69. doi: 10.1016/j.canlet.2019.02.001. Epub 2019 Feb 8
[PubMed PMID: 30742944]
Level 3 (low-level) evidence
[48]
Dreinhöfer KE, Rydholm A, Bauer HC, Kreicbergs A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. The Journal of bone and joint surgery. British volume. 1995 Mar:77(2):189-93
[PubMed PMID: 7706330]
Level 3 (low-level) evidence
[49]
Rock M. Curettage of giant cell tumor of bone. Factors influencing local recurrences and metastasis. La Chirurgia degli organi di movimento. 1990:75(1 Suppl):204-5
[PubMed PMID: 2249533]
[50]
Miller G, Bettelli G, Fabbri N, Capanna R. Curettage of giant cell tumor of bone. Introduction--material and methods. La Chirurgia degli organi di movimento. 1990:75(1 Suppl):203
[PubMed PMID: 2249532]
[51]
Lausten GS, Jensen PK, Schiødt T, Lund B. Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. International orthopaedics. 1996:20(3):172-6
[PubMed PMID: 8832321]
Level 3 (low-level) evidence
[52]
Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. The Journal of bone and joint surgery. American volume. 1987 Jan:69(1):106-14
[PubMed PMID: 3805057]
[53]
Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. The Journal of bone and joint surgery. American volume. 1982 Jun:64(5):755-61
[PubMed PMID: 7045129]
Level 3 (low-level) evidence
[54]
McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. The Journal of bone and joint surgery. American volume. 1986 Feb:68(2):235-42
[PubMed PMID: 3511063]
[55]
Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long-term follow-up. Japanese journal of clinical oncology. 1998 May:28(5):323-8
[PubMed PMID: 9703860]
[56]
van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovée JV, Hogendoorn PC, Gelderblom H. The clinical approach toward giant cell tumor of bone. The oncologist. 2014 May:19(5):550-61. doi: 10.1634/theoncologist.2013-0432. Epub 2014 Apr 9
[PubMed PMID: 24718514]
[57]
Puri A, Agarwal M. Treatment of giant cell tumor of bone: Current concepts. Indian journal of orthopaedics. 2007 Apr:41(2):101-8. doi: 10.4103/0019-5413.32039. Epub
[PubMed PMID: 21139760]
[58]
Fujimoto N, Nakagawa K, Seichi A, Terahara A, Tago M, Aoki Y, Hosoi Y, Ohtomo K. A new bisphosphonate treatment option for giant cell tumors. Oncology reports. 2001 May-Jun:8(3):643-7
[PubMed PMID: 11295095]
[59]
Chang SS, Suratwala SJ, Jung KM, Doppelt JD, Zhang HZ, Blaine TA, Kim TW, Winchester RJ, Lee FY. Bisphosphonates may reduce recurrence in giant cell tumor by inducing apoptosis. Clinical orthopaedics and related research. 2004 Sep:(426):103-9
[PubMed PMID: 15346059]
[60]
Zwolak P, Manivel JC, Jasinski P, Kirstein MN, Dudek AZ, Fisher J, Cheng EY. Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. The Journal of bone and joint surgery. American volume. 2010 Jan:92(1):162-8. doi: 10.2106/JBJS.H.01679. Epub
[PubMed PMID: 20048108]
[61]
Greenberg DD, Lee FY. Bisphosphonate-loaded Bone Cement as a Local Adjuvant Therapy for Giant Cell Tumor of Bone: A 1 to 12-Year Follow-up Study. American journal of clinical oncology. 2019 Mar:42(3):231-237. doi: 10.1097/COC.0000000000000504. Epub
[PubMed PMID: 30811352]
[62]
Xiang F, Liu H, Deng J, Ma W, Chen Y. Progress on Denosumab Use in Giant Cell Tumor of Bone: Dose and Duration of Therapy. Cancers. 2022 Nov 23:14(23):. doi: 10.3390/cancers14235758. Epub 2022 Nov 23
[PubMed PMID: 36497239]
[63]
Hindiskere S, Errani C, Doddarangappa S, Ramaswamy V, Rai M, Chinder PS. Is a Short-course of Preoperative Denosumab as Effective as Prolonged Therapy for Giant Cell Tumor of Bone? Clinical orthopaedics and related research. 2020 Nov:478(11):2522-2533. doi: 10.1097/CORR.0000000000001285. Epub
[PubMed PMID: 32401001]
[64]
Mahdal M, Neradil J, Mudry P, Paukovcekova S, Staniczkova Zambo I, Urban J, Macsek P, Pazourek L, Tomas T, Veselska R. New Target for Precision Medicine Treatment of Giant-Cell Tumor of Bone: Sunitinib Is Effective in the Treatment of Neoplastic Stromal Cells with Activated PDGFRβ Signaling. Cancers. 2021 Jul 15:13(14):. doi: 10.3390/cancers13143543. Epub 2021 Jul 15
[PubMed PMID: 34298757]
[65]
Taniguchi Y, Yamamoto N, Hayashi K, Takeuchi A, Miwa S, Igarashi K, Higuchi T, Abe K, Yonezawa H, Araki Y, Morinaga S, Kamei J, Nugroho AE, Kaneda T, Morita H, Tsuchiya H. Anti-tumor Effects of Cyclolinopeptide on Giant-cell Tumor of the Bone. Anticancer research. 2019 Nov:39(11):6145-6153. doi: 10.21873/anticanres.13822. Epub
[PubMed PMID: 31704842]
[66]
Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Jan-Feb:33(1):197-211. doi: 10.1148/rg.331125089. Epub
[PubMed PMID: 23322837]
[67]
Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. The Journal of bone and joint surgery. British volume. 1998 Jan:80(1):43-7
[PubMed PMID: 9460951]
[68]
Chan CM, Adler Z, Reith JD, Gibbs CP Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. The Journal of bone and joint surgery. American volume. 2015 Mar 4:97(5):420-8. doi: 10.2106/JBJS.N.00678. Epub
[PubMed PMID: 25740033]
[69]
Domovitov SV, Healey JH. Primary malignant giant-cell tumor of bone has high survival rate. Annals of surgical oncology. 2010 Mar:17(3):694-701. doi: 10.1245/s10434-009-0803-z. Epub 2009 Nov 10
[PubMed PMID: 19902306]
[70]
Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. The Journal of bone and joint surgery. American volume. 1970 Jun:52(4):619-64
[PubMed PMID: 5479455]
Level 3 (low-level) evidence
[71]
Aoki J, Watanabe H, Shinozaki T, Takagishi K, Ishijima H, Oya N, Sato N, Inoue T, Endo K. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology. 2001 Jun:219(3):774-7
[PubMed PMID: 11376267]
[72]
Wülling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. Journal of cancer research and clinical oncology. 2001 Aug:127(8):467-74
[PubMed PMID: 11501745]
[73]
Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. Journal of cancer research and clinical oncology. 2008 Sep:134(9):969-78. doi: 10.1007/s00432-008-0370-x. Epub 2008 Mar 6
[PubMed PMID: 18322700]
Level 3 (low-level) evidence
[74]
Balke M, Ahrens H, Streitbuerger A, Koehler G, Winkelmann W, Gosheger G, Hardes J. Treatment options for recurrent giant cell tumors of bone. Journal of cancer research and clinical oncology. 2009 Jan:135(1):149-58. doi: 10.1007/s00432-008-0427-x. Epub 2008 Jun 3
[PubMed PMID: 18521629]
[75]
Vult von Steyern F, Bauer HC, Trovik C, Kivioja A, Bergh P, Holmberg Jörgensen P, Follerås G, Rydholm A, Scandinavian Sarcoma Group. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian Sarcoma Group study. The Journal of bone and joint surgery. British volume. 2006 Apr:88(4):531-5
[PubMed PMID: 16567792]
[76]
Feigenberg SJ, Marcus RB Jr, Zlotecki RA, Scarborough MT, Enneking WF. Whole-lung radiotherapy for giant cell tumors of bone with pulmonary metastases. Clinical orthopaedics and related research. 2002 Aug:(401):202-8
[PubMed PMID: 12151897]
[77]
Bertoni F, Present D, Enneking WF. Giant-cell tumor of bone with pulmonary metastases. The Journal of bone and joint surgery. American volume. 1985 Jul:67(6):890-900
[PubMed PMID: 4019539]
[78]
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clinical orthopaedics and related research. 1980 Nov-Dec:(153):106-20
[PubMed PMID: 7449206]
[79]
Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in Giant Cell Tumor of Bone: A Review of the Literature. Technology in cancer research & treatment. 2019 Jan 1:18():1533033819840000. doi: 10.1177/1533033819840000. Epub
[PubMed PMID: 30935298]
[80]
Rekhi B, Dave V. Giant cell tumor of bone: An update, including spectrum of pathological features, pathogenesis, molecular profile and the differential diagnoses. Histology and histopathology. 2023 Feb:38(2):139-153. doi: 10.14670/HH-18-486. Epub 2022 Jun 29
[PubMed PMID: 35766228]