Function
Benign variants are reported in EEGs with a prevalence of 2.19% to 3.4%.[1] These may have an epileptiform morphology but are not associated with epilepsy. These patterns have no corresponding ictal patterns, and they are not known to be indicative of epileptogenicity. These patterns can be easily misinterpreted, and, therefore, there is a need for a thorough knowledge of clinical electroencephalographers regarding them to avoid misdiagnosis and unnecessary treatment. These fall into six main types: benign sporadic sleep spikes (BSSS), wicket spikes, 14 and 6 Hz positive spikes, 6 Hz spike and waves, rhythmic temporal theta-burst of drowsiness (RTTD), and subclinical rhythmic electrographic discharge of adults (SREDA). This review will discuss their EEG features, patients’ demographic characteristics, and clinical relevance.
Issues of Concern
Benign Sporadic Sleep Spikes (BSSS)
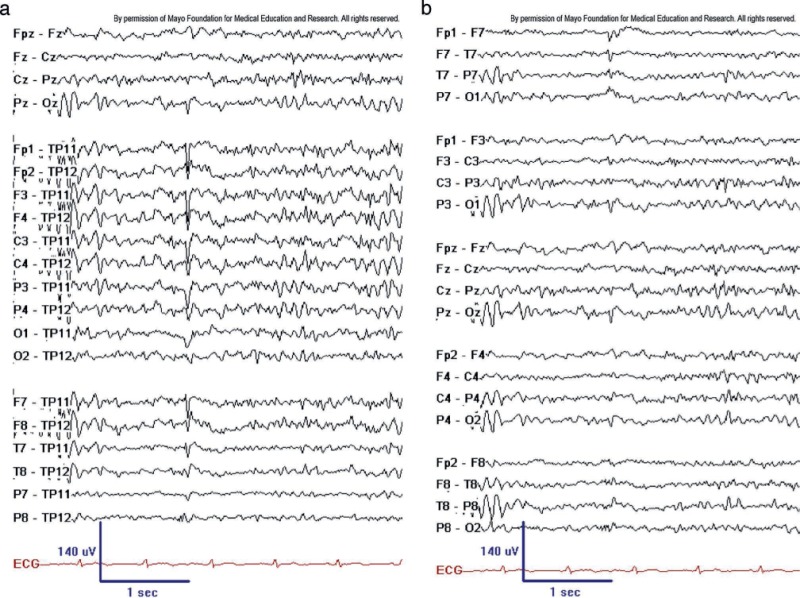
Benign sporadic sleep spikes (BSSS), also referred to as small sharp spikes or benign epileptiform transient of sleep (BETS), are low amplitude (<50 mV), brief (<50 ms), sharply contoured monophasic or biphasic electronegative spikes in theta or alpha frequency range (see figure 1). The identifying features of BSSS include their typically widespread horizontal dipole distribution that occurs in the absence of any apparent disruption of the ongoing background activities. However, SSS may occasionally be accompanied by an after-going slow-wave, and this slow-wave usually has a smaller amplitude relative to the spike.
BSSS occur in the broad temporal region and are characteristically difficult to localize precisely. BSSS often appear in both hemispheres either independently or bisynchronously and may have shifting asymmetries. They almost exclusively occur during drowsiness or light non-REM sleep. The appearance of stereotyped, isolated, small-amplitude spikes that do not disrupt the background and appear only in drowsiness or light sleep strongly suggests that the transient may be SSS. BSSS is one of the most common benign variants with a broad range of prevalence ranging from 1.85% up to 24% of scalp EEG recordings.[1] BSSS is usually seen in adults and is seldom seen in children.
The clinical significance of BSSS was thought to be a pattern associated with seizures. However, a study conducted by Molaie in 1991 concluded BSSS was likely normal variants.[2] A recent study with hippocampal implanted electrode investigations demonstrated that in some patients, their scalp SSS were time-locked to hippocampal epileptiform discharges and high-frequency oscillations. In that study, the author suspected that SSS might be an early indicator of a hippocampal pathologic condition.[3]
Wicket Spikes or Wicket Rhythms
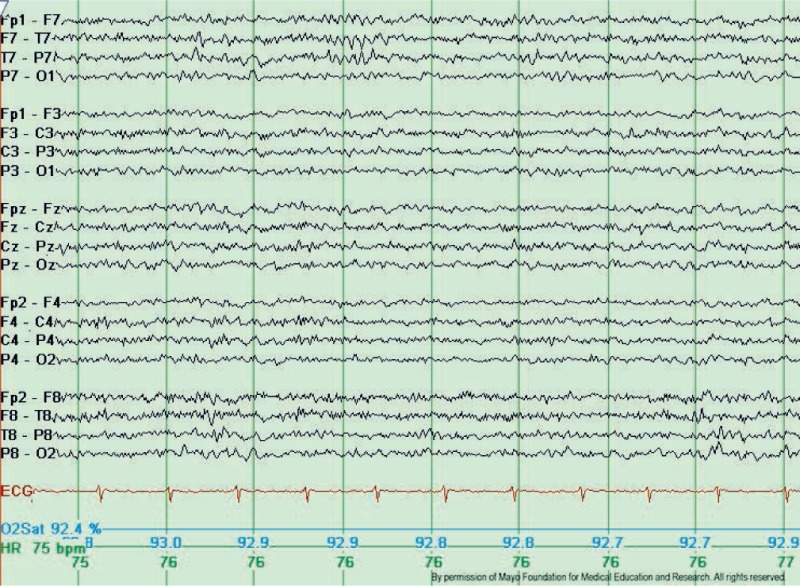
Wicket spikes or wicket rhythms are medium to high voltage, monophasic wave bursts in the range of theta or alpha range (6-11Hz). Wicket spikes occur in the anterior or middle temporal areas with a negative polarity, which usually evolves from the background as arcuate-shaped, brief (0.5-1 second) rhythmic discharges. Wicket spikes are most commonly seen in adults older than 30 years old during light sleep or in an individual whose background activity contains sharply contoured waveforms (see figure 2). Although wicket spikes were originally described as temporal in location, especially in the left temporal.[4] It is essential to be aware that similar isolated, sharply contoured waveforms may occur over any head region where there is a sharply contoured background activity.
The prevalence of wicket spikes is reported around 1% in several large studies. The rarity of this pattern, unilateral distribution, temporal location, and spike-like single-fragment appearance are the common reasons they are most often mistaken for interictal epileptiform discharges. Several features can be helpful to differentiate wicket spikes from interictal epileptiform discharges. Wicket spikes don't disturb the background activity and don't have an after-going slow wave. Wicket spikes tend to appear repetitively in trains of arch-shaped rhythms resembling mu rhythm.[5]
Wicket spikes have no association with epilepsy but are used to be believed to be more frequent in patients with cerebrovascular disease, dizziness or vertigo, and headache.[6][7]
6 Hz Spike and Wave Discharges ("Phantom" Spike-wave)
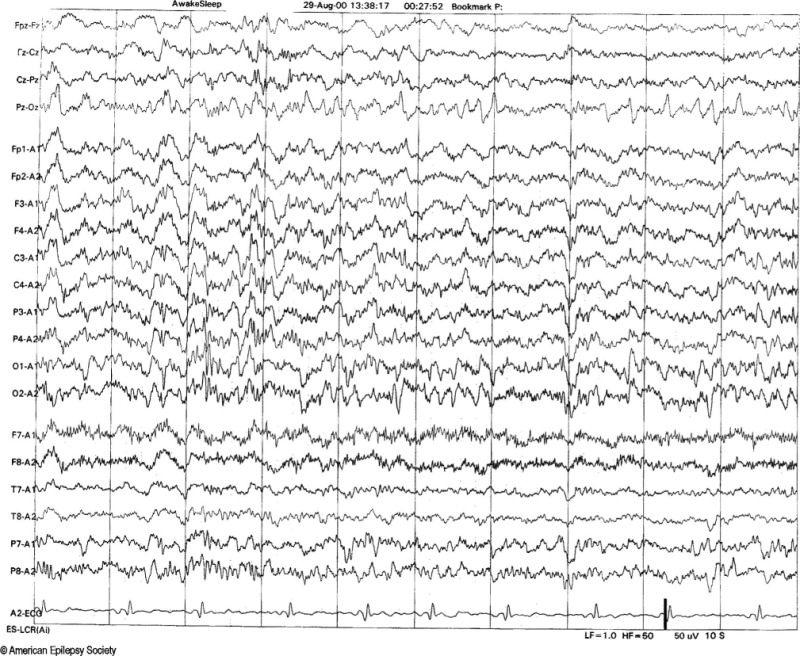
6 Hz spike and wave discharges consist of a 4 to 7 Hz repetitive spike and wave complex with a mitten-like morphology. It is also called "phantom" spike-wave since it has a relatively low amplitude (less than 40 mV), fast spike (less than 30 ms) followed by a 5 to 7 Hz wave of equal or greater amplitude (figure 3). 6 Hz spike and wave discharges occur in young adult bilaterally, synchronously and predominantly during relaxed wakefulness, drowsiness or light sleep, and occasionally in REM sleep.[8]
Two different forms of the 6 Hz spike and wave discharge have been described. The classical form is FOLD (Female Occipital Low-amplitude Drowsiness), which is relatively low in amplitude and is maximal over the posterior head regions. It is not associated with seizures. The second form is WHAM (Wake High-Amplitude Male), which is frontally dominant, often moderate or high amplitude.[9] This form of 6 Hz spike and wave discharge overlaps with abnormal generalized atypical spike and wave patterns with rapid repetition rates. WHAM is associated with epilepsy, especially when high amplitude spikes repetition rates are less than 5 Hz.
14 and 6 Hz Positive Spikes
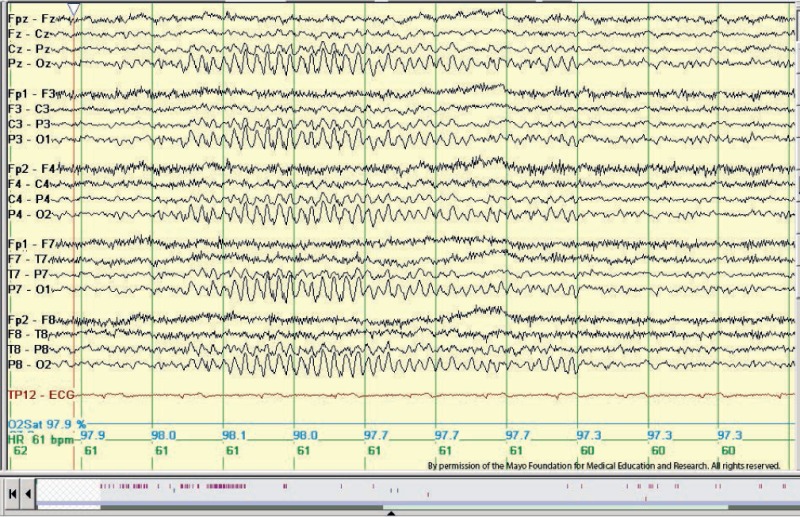
14 and 6 positive spikes are best appreciated by using long interelectrode distances and ear referential montages. 14 and 6 Hz positive spikes consist of brief runs (less than 1 second) of positive spikes at a rate of 14 Hz or 6 to 7 Hz. The amplitude is variable but rarely exceeds 75 mV (see figure 4). These bursts typically consist of "negative" arciform waveforms located over the posterior temporal head regions, with alternating "positive" spiky components. This pattern occurs either bisynchronously or unilaterally (independent over the two hemispheres at different times). The 14 Hz pattern often looks like a sleep spindle with a sharp positive phase, although it's location is quite different.
This benign variant pattern occurs in 20% to 60% of the normal population, predominantly in adolescents, especially during drowsiness and light non-REM sleep.[10] The 14-Hz positive spikes occur more frequently in adolescents. An unexplained finding is 14 and 6 Hz positive spikes occur in comatose patients with acute Reye syndrome, hepatic failure, postanoxic encephalopathy, or even head trauma.[11]
Rhythmic Mid-temporal Theta of Drowsiness (RMTDs)
Rhythmic mid-temporal theta of drowsiness or rhythmical mid-temporal discharges is also known as "psychomotor variant," which is so-called because they share similarities with epileptiform discharges that may be seen during psychomotor seizures. They are bursts of rhythmical sharp waves at the range of theta, lasting for a few seconds, which often have a top-notched by a small 10 to 12 Hz component. They occur in mid temporal regions, either on one side or on both, either independently or simultaneously. The key features distinguishing RMTDs from abnormal electrographic discharges or other benign normal variants are there is little variability in morphology and frequency during the bursts.[10] They often begin and end with a gradual increase and decrease of amplitude, but their overall frequency remains very stable throughout their appearance.
RMTDs are mostly seen in about 0.5% to 0.79% of young adults during their light sleep.[1] Currently, RMTDs are considered benign variants based on lacking associated clinical change, although previous studies once proposed that RMTDs were the least epileptogenic of temporal discharges.[12] Lin evaluated the source location and clinical significance of RMTDs by Magnetoencephalography (MEG), which indicates that the source of RMTDs activity is located in the fissural cortex of the posterior inferior temporal region and RMTD has no direct relation to the epileptogenic activity.[13]
Subclinical Rhythmic EEG Discharge of Adults (SREDA)
Subclinical rhythmic EEG discharges of adults are the least common of benign variants with a prevalence of 0.04% to 0.07%.[1][14] SREDA is characterized as symmetrical, diffuse, rhythmic monomorphic theta waves with sharp contours, maximally on the parietal and posterior temporal regions. In some cases, it is either asymmetric or unilateral. SREDA typically begins abruptly or is delayed 1 to several seconds after a single high amplitude mono- or bi-phasic sharp or slow-wave component. Once established, the pattern may consist of repetitive monophasic sharp waveforms in about 1 to 2 Hz and gradually evolve into a sustained sinusoidal 4 to 7 Hz pattern. This pattern may either end abruptly or gradually diminish and merge with the background. SREDA usually lasts about 40 to 80 seconds, but it may be shorter than 10 seconds or longer than several minutes.[10]
Like RMTDs, SREDA is likely to be misinterpreted as an ictal epileptiform pattern and become misleading in nonepileptic paroxysmal events. There are several distinctive features[15]: (1) SREDA occurs while the patient is awake; response testing can demonstrate that consciousness and mentation are preserved. (2) Its tendency to be present in subsequent EEGs, even when more than several years have elapsed between recordings. (3) Its relative lack of evolution in frequency, morphology, or distribution compared to most abnormal seizure patterns, and in some cases, the background may persist. (4) The absence of postictal EEG changes.
However, SREDA has come to be recognized as a wide spectrum of typical and atypical variants. Atypical features of SREDA consist predominantly of delta frequencies, notched waveforms, frontal or more focal distributions, asymmetric or unilateral, a more prolonged duration, and presence during sleep.[16] Atypical SERDA remains a challenge for epileptologists.
Although SREDA occurs mainly in elderly individuals during wakefulness, there are reports of this pattern seen in the younger adults during REM and non-REM stages of sleep or even children. Several scholars proposed the term SREDAC (subclinical rhythmic EEG discharge of adults and children), but currently, there are only 4 cases reported in total.[17]
The literature indicates that SREDA is difficult to associate with any specific condition or type of individual. Multiple reports concerning the presentation of SREDA in cases of vascular and nonvascular neurologic and non-neurologic diseases, such as migraine, syncope, transient ischemic attack, epilepsy, and the hemolytic uremic syndrome, were subsequently published.[18][19]
Clinical Significance
Electroencephalographers may misclassify benign variant EEG patterns as epileptiform discharges, resulting in unnecessary treatment or a delay in the diagnosis and appropriate treatment of other paroxysmal disorders. Cautious interpretation of EEG patterns, a careful review of clinical history, and demographic features usually help distinguish normal variant patterns from interictal epileptiform discharges or seizures. When nonspecific transients occur during an EEG finding, it may not be possible to determine whether a transient is an epileptiform discharge or a benign variant. It is best not to “overcall” a finding as being abnormal, and to place the possible finding in the context of the clinical history.
Enhancing Healthcare Team Outcomes
Electroencephalogram (EEG) is essential in the diagnosis of seizures and psychogenic non-epileptic spells. It is important to distinguish between pathologic activity and similar-appearing normal physiologic findings. Various terminologies are used, such as: "normal variants," "benign variants," "pseudo-epileptiform patterns," or "epileptiform patterns without proven relation to seizures." Benign epileptiform variants in an electroencephalogram should be identified to assist primary care providers, neurologists, and nurses provide optimal care.
Recognition of these EEG waveforms should be part of an interprofessional team approach to diagnosis and interpretation. While the neurologist has the ultimate say in diagnosis, input from other clinicians, NPS and PAs, and even rad techs can be informative to arrive at an accurate clinical picture, which will improve patient care and outcomes. [Level 5]