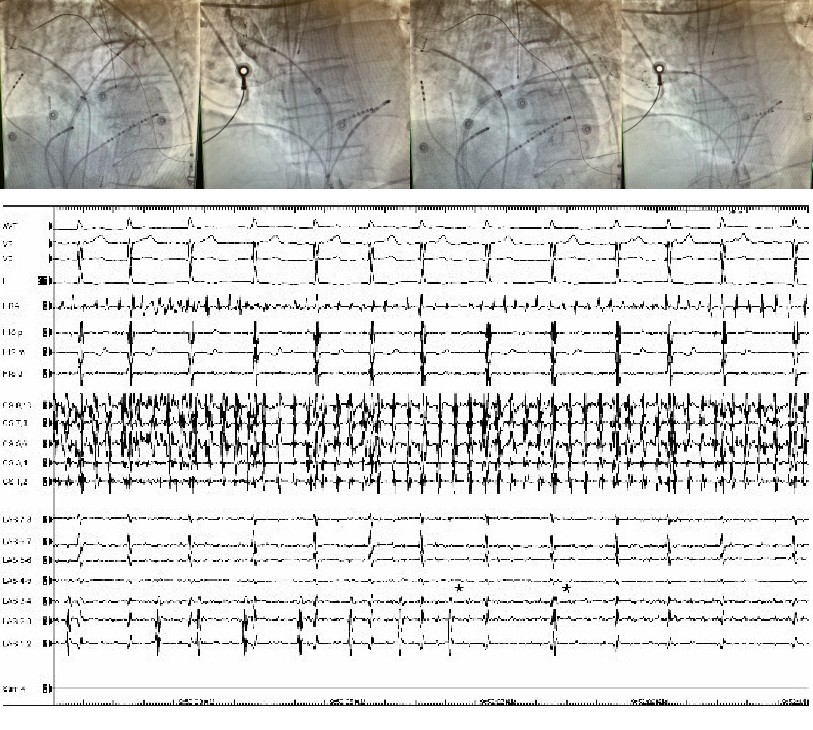
[1]
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014 Dec 2:64(21):e1-76. doi: 10.1016/j.jacc.2014.03.022. Epub 2014 Mar 28
[PubMed PMID: 24685669]
Level 1 (high-level) evidence
[2]
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11:114(2):119-25
[PubMed PMID: 16818816]
[3]
Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet (London, England). 2009 Feb 28:373(9665):739-45. doi: 10.1016/S0140-6736(09)60443-8. Epub
[PubMed PMID: 19249635]
[4]
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England journal of medicine. 1998 Sep 3:339(10):659-66
[PubMed PMID: 9725923]
[5]
Brick AV, Braile DM. Surgical Ablation of Atrial Fibrillation Using Energy Sources. Brazilian journal of cardiovascular surgery. 2015 Nov-Dec:30(6):636-43. doi: 10.5935/1678-9741.20150078. Epub
[PubMed PMID: 26934404]
[6]
Garg J, Chaudhary R, Palaniswamy C, Shah N, Krishnamoorthy P, Bozorgnia B, Natale A. Cryoballoon versus Radiofrequency Ablation for Atrial Fibrillation: A Meta-analysis of 16 Clinical Trials. Journal of atrial fibrillation. 2016 Oct-Nov:9(3):1429. doi: 10.4022/jafib.1429. Epub 2016 Oct 31
[PubMed PMID: 28496925]
Level 1 (high-level) evidence
[7]
Goel R, Anderson K, Slaton J, Schmidlin F, Vercellotti G, Belcher J, Bischof JC. Adjuvant approaches to enhance cryosurgery. Journal of biomechanical engineering. 2009 Jul:131(7):074003. doi: 10.1115/1.3156804. Epub
[PubMed PMID: 19640135]
[8]
Barnett AS, Bahnson TD, Piccini JP. Recent Advances in Lesion Formation for Catheter Ablation of Atrial Fibrillation. Circulation. Arrhythmia and electrophysiology. 2016 May:9(5):. doi: 10.1161/CIRCEP.115.003299. Epub
[PubMed PMID: 27103088]
Level 3 (low-level) evidence
[9]
Wittkampf FH, Derksen R, Wever EF, Simmers TA, Boersma LV, Vonken EP, Velthuis BK, Sreeram N, Rensing BJ, Cramer MJ. Technique of pulmonary vein isolation by catheter ablation. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2002 May:10(5):241-244
[PubMed PMID: 25696100]
[10]
Katz-Agranov N, Nevah Rubin MI. Severe esophageal injury after radiofrequency ablation - a deadly complication. World journal of gastroenterology. 2017 May 14:23(18):3374-3378. doi: 10.3748/wjg.v23.i18.3374. Epub
[PubMed PMID: 28566899]
[11]
Ozcan C, Ruskin J, Mansour M. Cryoballoon catheter ablation in atrial fibrillation. Cardiology research and practice. 2011:2011():256347. doi: 10.4061/2011/256347. Epub 2011 Jun 20
[PubMed PMID: 21747987]
[12]
Leila R, Raluca P, Yves G, Dirk S, Bruno S. Cryoablation Versus Radiofrequency Ablation in AVNRT: Same Goal, Different Strategy. Journal of atrial fibrillation. 2015 Jun-Jul:8(1):1220. doi: 10.4022/jafib.1220. Epub 2015 Jun 30
[PubMed PMID: 27957174]
[13]
Kuck K, Brugada J, Albenque J. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation. The New England journal of medicine. 2016 Sep 15:375(11):1100-1. doi: 10.1056/NEJMc1609160. Epub
[PubMed PMID: 27626535]
[14]
Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, Angkeow P, LaCorte J, Bluemke D, Berger R, Halperin HR, Calkins H. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003 Apr 22:107(15):2004-10
[PubMed PMID: 12681994]
[15]
Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966 Sep:34(3):412-22
[PubMed PMID: 5922708]
[16]
Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts-Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the "second factor". Journal of the American College of Cardiology. 2009 Apr 7:53(14):1182-91. doi: 10.1016/j.jacc.2008.11.054. Epub
[PubMed PMID: 19341858]
[17]
Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circulation research. 2014 Apr 25:114(9):1500-15. doi: 10.1161/CIRCRESAHA.114.303772. Epub
[PubMed PMID: 24763467]
[18]
Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Comprehensive Physiology. 2015 Apr:5(2):649-65. doi: 10.1002/cphy.c140047. Epub
[PubMed PMID: 25880508]
Level 3 (low-level) evidence
[19]
Calkins H,Hindricks G,Cappato R,Kim YH,Saad EB,Aguinaga L,Akar JG,Badhwar V,Brugada J,Camm J,Chen PS,Chen SA,Chung MK,Nielsen JC,Curtis AB,Davies DW,Day JD,d'Avila A,de Groot NMSN,Di Biase L,Duytschaever M,Edgerton JR,Ellenbogen KA,Ellinor PT,Ernst S,Fenelon G,Gerstenfeld EP,Haines DE,Haissaguerre M,Helm RH,Hylek E,Jackman WM,Jalife J,Kalman JM,Kautzner J,Kottkamp H,Kuck KH,Kumagai K,Lee R,Lewalter T,Lindsay BD,Macle L,Mansour M,Marchlinski FE,Michaud GF,Nakagawa H,Natale A,Nattel S,Okumura K,Packer D,Pokushalov E,Reynolds MR,Sanders P,Scanavacca M,Schilling R,Tondo C,Tsao HM,Verma A,Wilber DJ,Yamane T, 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Journal of arrhythmia. 2017 Oct
[PubMed PMID: 29021841]
Level 3 (low-level) evidence
[20]
Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, Poole JE, Mascette A, Rosenberg Y, Jeffries N, Al-Khalidi HR, Lee KL, CABANA Investigators. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. American heart journal. 2018 May:199():192-199. doi: 10.1016/j.ahj.2018.02.015. Epub 2018 Mar 7
[PubMed PMID: 29754661]
[21]
Liu X, Palmer J. Outcomes of 200 consecutive, fluoroless atrial fibrillation ablations using a new technique. Pacing and clinical electrophysiology : PACE. 2018 Nov:41(11):1404-1411. doi: 10.1111/pace.13492. Epub 2018 Sep 19
[PubMed PMID: 30194724]
[22]
Fürnkranz A, Köster I, Chun KR, Metzner A, Mathew S, Konstantinidou M, Ouyang F, Kuck KH. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart rhythm. 2011 Jun:8(6):821-5. doi: 10.1016/j.hrthm.2011.01.044. Epub 2011 Apr 11
[PubMed PMID: 21315836]
[23]
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005 Mar 8:111(9):1100-5
[PubMed PMID: 15723973]
Level 3 (low-level) evidence
[24]
Parikh V, Kowalski M. Comparison of Phrenic Nerve Injury during Atrial Fibrillation Ablation between Different Modalities, Pathophysiology and Management. Journal of atrial fibrillation. 2015 Dec:8(4):1314. doi: 10.4022/jafib.1314. Epub 2015 Dec 31
[PubMed PMID: 27957229]
[25]
Canpolat U, Kocyigit D, Aytemir K. Complications of Atrial Fibrillation Cryoablation. Journal of atrial fibrillation. 2017 Dec:10(4):1620. doi: 10.4022/jafib.1620. Epub 2017 Dec 31
[PubMed PMID: 29487676]
[26]
Mujović N, Marinković M, Lenarczyk R, Tilz R, Potpara TS. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Advances in therapy. 2017 Aug:34(8):1897-1917. doi: 10.1007/s12325-017-0590-z. Epub 2017 Jul 21
[PubMed PMID: 28733782]
Level 3 (low-level) evidence
[27]
Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW, Ruskin JN, STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. Journal of the American College of Cardiology. 2013 Apr 23:61(16):1713-23. doi: 10.1016/j.jacc.2012.11.064. Epub 2013 Mar 21
[PubMed PMID: 23500312]
[28]
Andrade JG, Dubuc M, Guerra PG, Macle L, Rivard L, Roy D, Talajic M, Thibault B, Khairy P. Cryoballoon ablation for atrial fibrillation. Indian pacing and electrophysiology journal. 2012 Mar:12(2):39-53
[PubMed PMID: 22557842]
[29]
Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circulation. Arrhythmia and electrophysiology. 2009 Aug:2(4):349-61. doi: 10.1161/CIRCEP.108.824789. Epub 2009 Jun 2
[PubMed PMID: 19808490]
Level 1 (high-level) evidence
[30]
Kuck KH, Fürnkranz A. Cryoballoon ablation of atrial fibrillation. Journal of cardiovascular electrophysiology. 2010 Dec:21(12):1427-31. doi: 10.1111/j.1540-8167.2010.01944.x. Epub 2010 Nov 23
[PubMed PMID: 21091966]
[31]
Andrade JG, Khairy P, Guerra PG, Deyell MW, Rivard L, Macle L, Thibault B, Talajic M, Roy D, Dubuc M. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart rhythm. 2011 Sep:8(9):1444-51. doi: 10.1016/j.hrthm.2011.03.050. Epub 2011 Mar 30
[PubMed PMID: 21457789]
Level 1 (high-level) evidence
[32]
Kubala M, Hermida JS, Nadji G, Quenum S, Traulle S, Jarry G. Normal pulmonary veins anatomy is associated with better AF-free survival after cryoablation as compared to atypical anatomy with common left pulmonary vein. Pacing and clinical electrophysiology : PACE. 2011 Jul:34(7):837-43. doi: 10.1111/j.1540-8159.2011.03070.x. Epub 2011 Mar 21
[PubMed PMID: 21418249]
[33]
Khoueiry Z, Albenque JP, Providencia R, Combes S, Combes N, Jourda F, Sousa PA, Cardin C, Pasquie JL, Cung TT, Massin F, Marijon E, Boveda S. Outcomes after cryoablation vs. radiofrequency in patients with paroxysmal atrial fibrillation: impact of pulmonary veins anatomy. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 Sep:18(9):1343-51. doi: 10.1093/europace/euv419. Epub 2016 Jan 27
[PubMed PMID: 26817755]
[34]
Sorgente A, Chierchia GB, de Asmundis C, Sarkozy A, Namdar M, Capulzini L, Yazaki Y, Müller-Burri SA, Bayrak F, Brugada P. Pulmonary vein ostium shape and orientation as possible predictors of occlusion in patients with drug-refractory paroxysmal atrial fibrillation undergoing cryoballoon ablation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011 Feb:13(2):205-12. doi: 10.1093/europace/euq388. Epub 2010 Oct 25
[PubMed PMID: 20974756]